Figure 9.
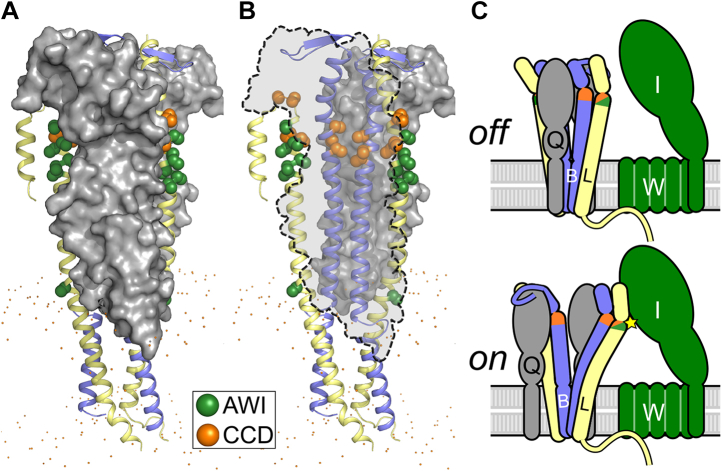
A structural shift in the coiled coil of FtsLB may be involved in its activation.A, preliminary model of the FtsQLB complex, obtained by aligning the region of FtsB that is in common between the Y-model and the crystal structure of FtsQ (Protein Data Bank code: 6H9O). In this model, the four helices of the coiled coil of FtsLB form a flat bundle sandwiched between the two periplasmic domains of FtsQ (gray). B, see-through representation of the same model, with one of the two FtsQ subunits removed and represented by a dashed outline. The positions of the CCD and AWI domains of FtsB and FtsL are highlighted as orange and green spheres, respectively. FtsB is nearly completely buried. FtsL is more solvent exposed, and its AWI positions occur on a solvent-facing surface. C, schematic representation of a potential model for FtsLB activation. A structural shift involving the coiled coil of FtsLB (potentially involving the separation of the two branches) exposes the AWI region of FtsL to a hypothesized protein–protein interaction with FtsI (star). This transition may be favored by destabilization of the coiled-coil region. AWI, activation of FtsWI; CCD, constriction control domain.