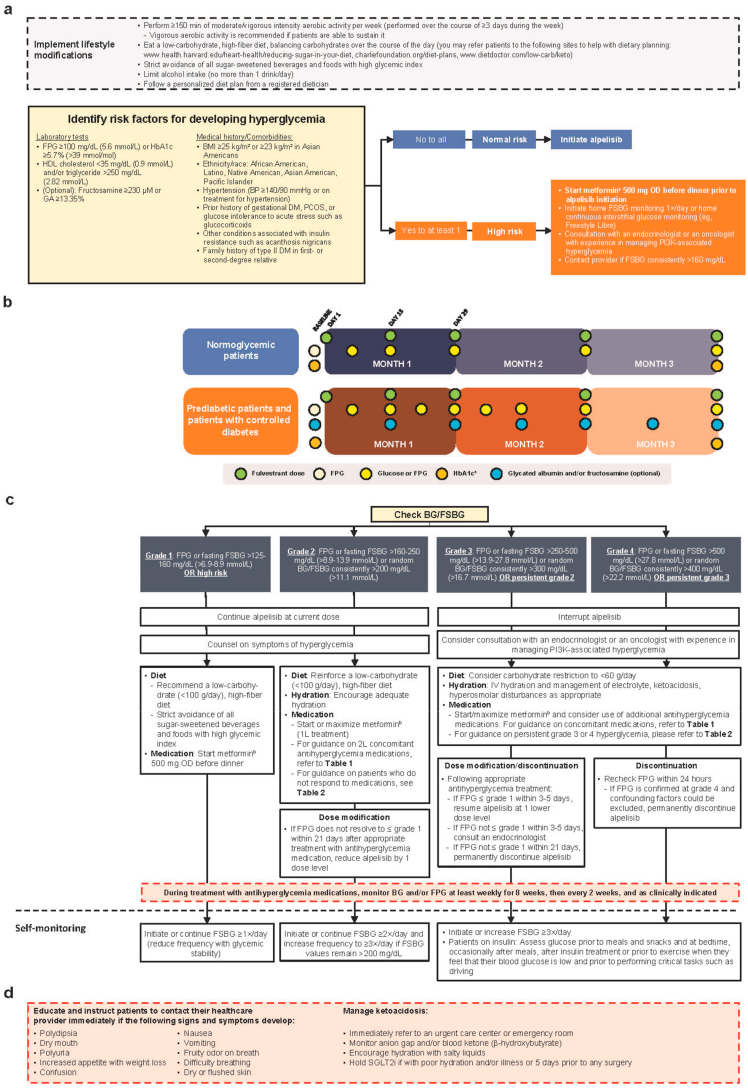
Fig. 1.
Management of alpelisib-associated hyperglycemia: [a] Lifestyle modifications and baseline glucose monitoring recommendations prior to initiating alpelisib treatment; [b] monitoring during alpelisib treatment; [c] hyperglycemia management recommendations; and self-monitoring guidelines during alpelisib treatment (severity based on CTCAE v4.03) [d] complications of hyperglycemia including ketoacidosis [10,12,[14], [15], [16]]. 1 L, first-line; 2 L, second-line; BG, blood glucose; BID, twice daily; BMI, body mass index; BP, blood pressure; CTCAE, Common Terminology Criteria for Adverse Events; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; FSBG, fingerstick blood glucose; GA, glycated albumin; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; IV, intravenous; OD, once daily; PCOS, polycystic ovarian syndrome; PI3K, phosphatidylinositol-3-kinase; SGLT2i, sodium-glucose co-transporter 2 inhibitor. aMay be done more frequently as clinically indicated. bAssess eGFR prior to initiation of metformin; do not initiate metformin in patients with eGFR 30–45 mL/min/1.73 m2 but consider 50% dose reduction in patients already on metformin and monitor renal function every 3 months. Metformin is contraindicated in patients with eGFR <30 mL/min/1.73 m2 (see Table 1 [12,[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]] for further details) [18,19]. Initiate metformin at 500 mg OD before dinner, increasing to 500 mg BID (before breakfast and dinner) as tolerated. May increase to 500 mg at before breakfast then 1000 mg before dinner, and then to 1000 mg BID if tolerated. If not tolerated, reduce to prior tolerated dose.