Table 1.
Top: Selected optimization experiments illustrating the influence of TFE and halide counterion in the enantioselective transfer hydrogenative coupling of alkyne 1a and ethanol 2a to form the product of carbonyl anti-(α-aryl)allylation 3a. Bottom: Effect of TFE on the ruthenium-JOSIPHOS catalyzed C-C coupling of alkynes 1b-1e with alcohols 2b-2e to form adducts 4a-4d.a
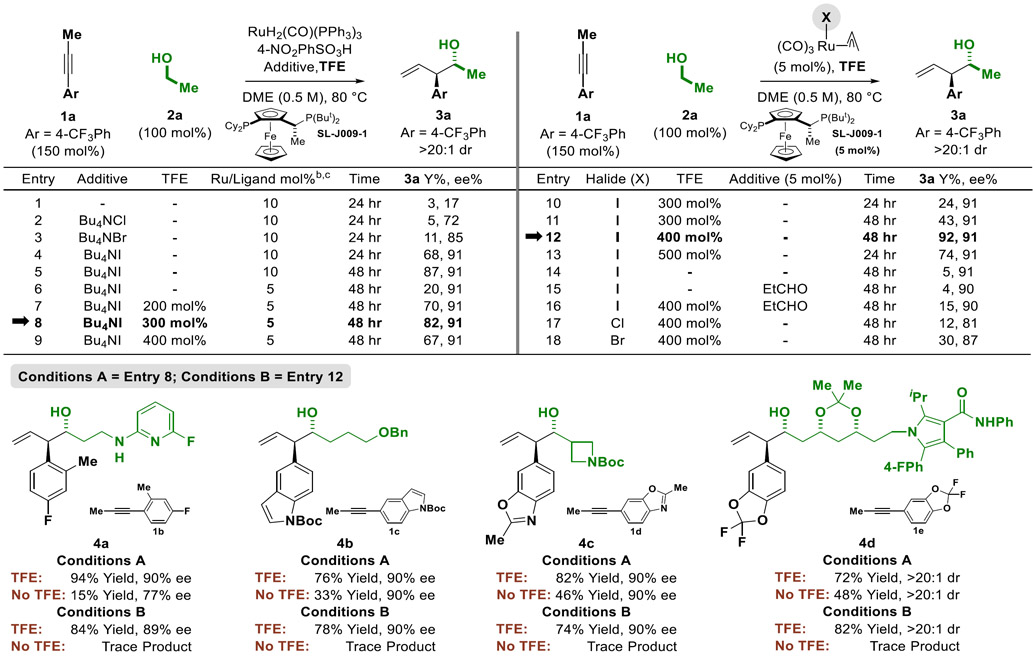
|
Yields of material isolated by silica gel chromatography. Enantioselectivities were determined by chiral stationary phase HPLC analysis. Diastereoselectivities were determined by 1H NMR analysis of crude reaction mixtures.
Entries 1-5: H2Ru(CO)(PPh3)3 (10 mol%), SL-J009-1 (10 mol%), Bu4NI (20 mol%) and 4-NO2PhSO3H (10 mol%).
Entries 6-9, H2Ru(CO)(PPh3)3 (5 mol%), SL-J009-1 (5 mol%), Bu4NI (10 mol%) and 4-NO2PhSO3H (5 mol%).
