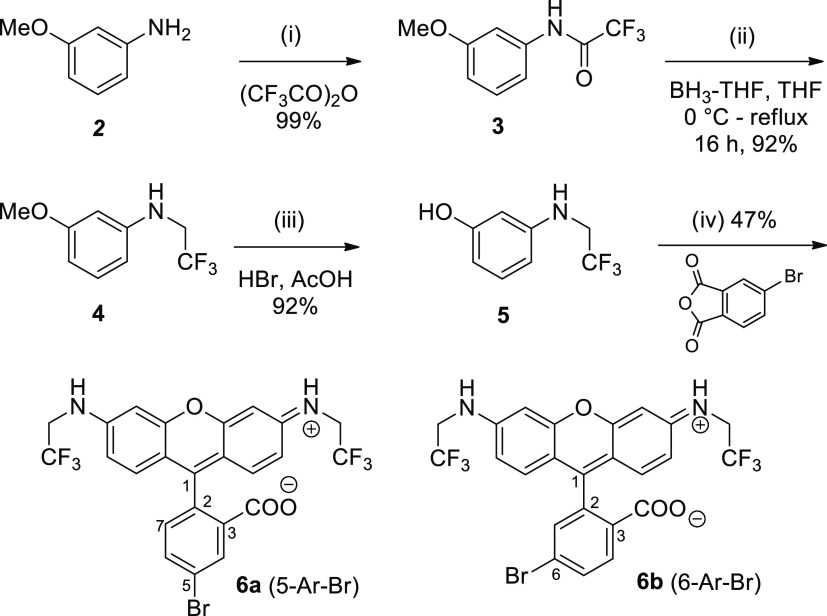
Scheme 2. Synthesis of Regioisomeric Bromorhodamines 6a and 6b Containing N,N′-Bis(2,2,2-trifluoroethyl) Groups.
Conditions: (i) pyridine, CH2Cl2, rt, overnight; (iii) 48% aq. HBr, AcOH, reflux, 6 h; (iv) method A: 160 °C, 3 h; addition of 5 (2nd equiv), 85% aq. H3PO4, 160 °C, 3 h (47%); method B: 1,2-dichlorobenzene, 160 °C, 3 h, addition of 5 (2nd equiv), 160 °C, 3 h (31%).