Table 2.
Features of the multi kinase inhibitors approved by the Food and Drug Administration (FDA) from 2011 to 2021. The order of drugs is tabulated in order of most recent to the oldest registration date.
| No. | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approved Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Fedratinib | INREBIC Celgene Corporation, Summit, NJ, USA |
FDA: 16 August 2019 EMA: 8 February 2021 |
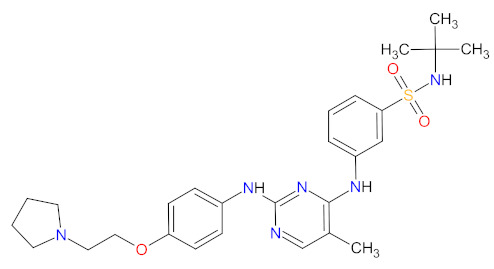
|
JAK2 2 | Oral | Myelofibrosis | Diarrhea, nausea, vomiting, constipation, anemia, thrombocytopenia | [66] 1, [67,68] |
| 2 | Gilteritinib | XOSPATA Astellas Pharma US, Inc., Northbrook, IL, USA |
FDA: 28 November 2018 EMA: 24 October 2019 |
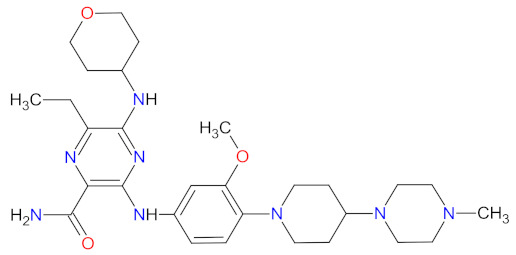
|
FLT3 3, AXL 4, ALK 5 |
Oral | Acute Myeloid Leukemia | Myalgia, arthralgia, increased levels of transaminases, fatigue, malaise, fever, diarrhea, dyspnea, edema, rash, pneumonia, sepsis, renal impairment | [69,70] |
| 3 | Midostaurin | RYDAPT Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA |
FDA: 28 April 2017 EMA: 18 September 2017 |
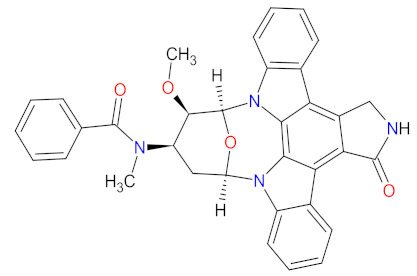
|
c-Kit 6, PDGFRA 7, PDGFRB 8, FLT3 3, PKC 9, CDK1 10, SYK 11, VEGFR-2 12 |
Oral | Acute Myeloid Leukemia, Cutaneous Mastocytosis | Febrile neutropenia, nausea, vomiting, diarrhea, edema, mucositis, headache, device-related infection, abdominal pain, fatigue, pyrexia, dyspnea, musculoskeletal pain, constipation, epistaxis, upper respiratory tract infection, petechial, hyperglycemia, | [71,72] |
| 4 | Ponatinib | ICLUSIG Ariad Pharmaceuticals, Inc., Cambridge, MA, USA |
FDA: 14 December 2012 EMA: 1 July 2013 |
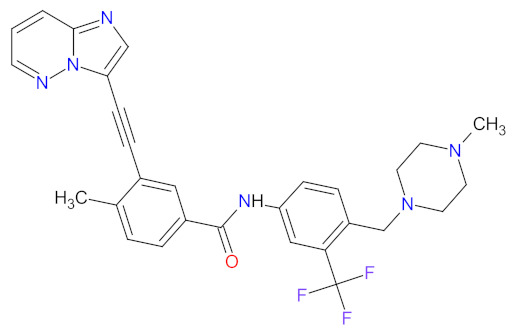
|
BCR-ABL 13, VEGFRs 14, FGFRs 15, PDGFRs 16, RET 17, c-Kit 6, TIE2 18, FLT3 3 |
Oral | Chronic Myelogenous Leukemia, Acute Lymphoblastic Leukemia |
Hypertension, cardiac failure, abdominal pain, constipation, diarrhea, oral mucositis, febrile neutropenia, fatigue, pneumonia, headache, peripheral neuropathy, dizziness, pleural effusion, cough, dyspnea, rush, dry skin, arthralgia, myalgia, spasms, decreased appetite, edema, weight loss, insomnia | [73,74] |
| 5 | Ruxolitinib | JAKAFI Incyte Corporation, Wilmington, DE, USA |
FDA: 16 November 2011 EMA: 23 August 2012 |
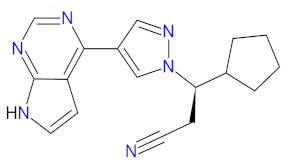
|
JAK1 19, JAK2 2 | Oral | Myelofibrosis, Polycythemia Vera, Graft-versus-host disease |
Anemia, thrombocytopenia, neutropenia | [75,76] |
1 Wrong chemical structure of the drug is given in the reference. 2 JAK2: Janus kinase 2. 3 FLT3: FMS-like tyrosine kinase-3. 4 AXL: AXL receptor tyrosine kinase. 5 ALK: anaplastic lymphoma kinase. 6 c-Kit: mast/stem cell growth factor receptor. 7 PDGFRA: platelet-derived growth factor receptor α. 8 PDGFRB: platelet-derived growth factor receptor β. 9 PKC: protein kinase C. 10 CDK1: cyclin-dependent kinase 1. 11 SYK: spleen tyrosine kinase. 12 VEGFR-2: vascular endothelial growth factor receptor-2. 13 BCR-ABL: BCR-ABL fusion protein. 14 VEGFRs: vascular endothelial growth factor receptors. 15 FGFRs: fibroblast growth factor receptors. 16 PDGFRs: platelet-derived growth factor receptors. 17 RET: receptor tyrosine kinase rearranged during transfection. 18 TIE2: tunica interna endothelial cell kinase 2. 19 JAK1: Janus kinase 1.
