Table 3.
Features of the phosphatidylinositol-3 kinase (PI3K) inhibitors approved by the Food and Drug Administration (FDA) from 2011 to 2021. The order of drugs is tabulated in order of most recent to oldest registration date.
| No. | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approved Date | Structure | Molecular Target |
Route of Administration |
Indication | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Duvelisib | COPIKTRA Verastem, Inc. Needham, MA, USA | FDA: 24 September 2018 EMA: 19 May 2021 |
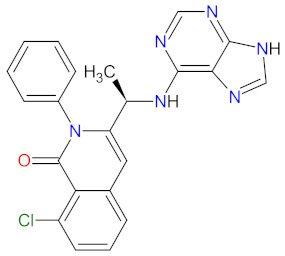
|
PI3K-δ 1, PI3K-γ 2 | Oral | Chronic Lymphocytic Leukemia, Follicular Lymphoma | Neutropenia, thrombocytopenia, anemia, diarrhea, pyrexia, nausea, vomiting, anorexia |
[84,85] |
| 2 | Copanlisib | ALIQOPA Bayer HealthCare Pharmaceuticals Inc., HanoverWhippany, NJ, USA |
FDA: 14 September 2017 EMA: Not approved |
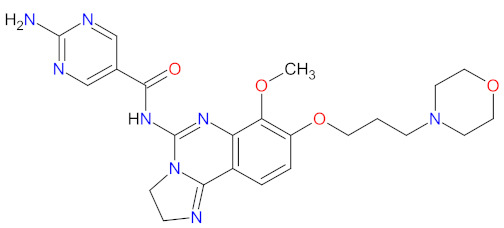
|
PI3K-α 3, PI3K-δ 1 | Intravenous infusion | Follicular Lymphoma |
Hyperglycemia, hypertension, infections, neutropenia |
[86,87] |
| 3 | Idelalisib | ZYDELIG Gilead Sciences, Inc., Foster City, CA, USA |
FDA: 23 July 2014 EMA: 18 September 2014 |
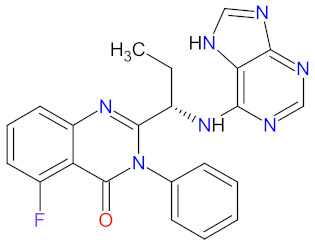
|
PI3K-δ 1 | Oral | Chronic Lymphocytic Leukemia, Follicular Lymphoma | Diarrhea, nausea, vomiting, fatigue, headache, pneumonia, chill, dyspnea, rash, neutropenia, pyrexia, sepsis, decreased neutrophil count, hypertriglyceridemia, hyperglycemia, elevated alanine and aspartate transaminases |
[88,89] |
1 PI3K-δ: phosphatidylinositol 3-kinase delta. 2 PI3K-γ: phosphatidylinositol 3-kinase gamma. 3 PI3K-α: phosphatidylinositol 3-kinase alpha.
