Table 13.
Features of the antibody-drug conjugates approved by the Food and Drug Administration (FDA) from 2011 to 2021. The order of drugs is tabulated in order of most recent to oldest registration date.
| No. | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approved Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Belantamab mafodotin-blmf | BLENREP GlaxoSmithKline, Brentford, England |
FDA: 5 August 2020 EMA: 25 August 2020 |
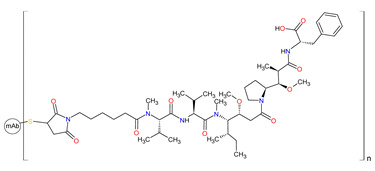
|
BCMA 1 | Intravenous | Multiple Myeloma | Ocular toxicity, thrombocytopenia, infusion-related reactions, gastrointestinal disorders, pyrexia, fatigue | [265,266] |
| 2 | Polatuzumab vedotin-piiq | POLIVY Genentech, Inc., South San Francisco, CA, USA | FDA: 10 June 2019 EMA: 16 January 2020 |
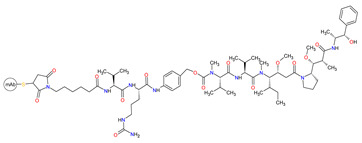
|
CD79b 2 | Intravenous | Diffuse Large B-Cell Lymphoma | Cytopenias | [262,267,268] |
| 3 | Inotuzumab ozogamicin | BESPONSA Pfizer Inc., New York, NY, USA | FDA: 17 August 2017 EMA: 29 June 2017 |
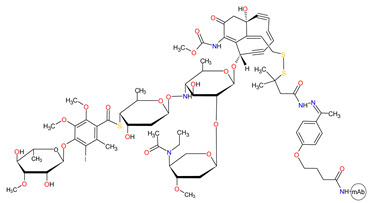
|
CD22 3 | Intravenous | Acute Lymphoblastic Leukemia | Cytopenias (including febrile neutropenia), infections, nausea, pyrexia, abnormal liver function and venoocclusive liver disease | [269,270] |
| 4 | Brentuximab vedotin | ADCETRIS Seattle Genetics, Inc., Bothell, WA, USA | FDA: 19 August 2011 EMA: 25 October 2012 |
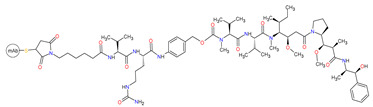
|
CD30 4 | Intravenous | Lymphoma, Hodgkin’s Lymphoma, Mycosis Fungoides | Neutropenia, anemia, peripheral sensory neuropathy, nausea, fatigue, constipation, diarrhea, vomiting, and pyrexia | [260,271,272] |
1 BCMA: B-cell maturation antigen. 2 CD79b: cluster of differentiation 79b. 3 CD22: cluster of differentiation 22. 4 CD30: cluster of differentiation 30.
