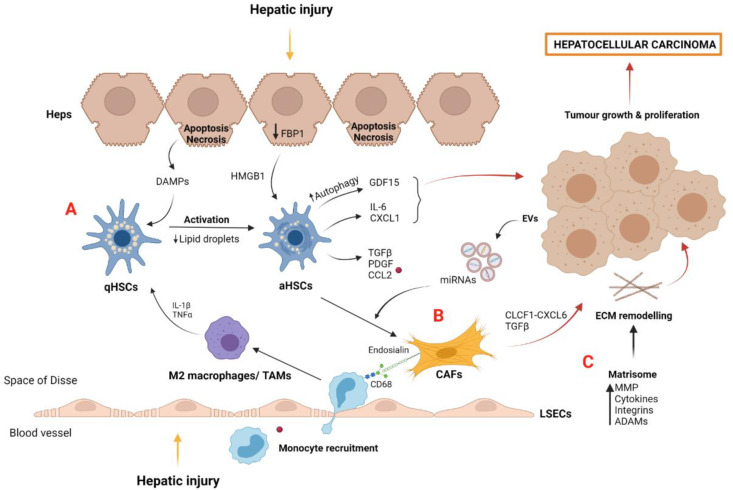
Figure 3.
Myofibroblast-derived cells and matrisome contribution to tumor microenvironment and HCC development: (A) Quiescent HSCs become activated by DAMPs released by apoptotic hepatocytes and by IL-1β and TNFα secreted by polarized M2 macrophages after hepatic injury. aHSCs induce the secretion of proinflammatory growth factors and chemokines such as CCL2, which is recognized by macrophage CCR2, perpetuating liver damage and tumor growth. aHSCs, induced by the loss of hepatocyte FBP1, secrete cytokines such as IL-6 and CXCL1, which are involved in liver tumorigenesis. Growth factor GDF15, induced by aHSC autophagy, also contributes to hepatocarcinoma. (B) CAFs are derived from activated myofibroblasts such as aHSCs. Diverse growth factors and miRNAs contained in EVs and released from cancer cells act as positive stimuli for CAF differentiation. CAFs promote tumor development via the CLCF1–CXCL6/TGFβ signaling pathway and induce the recruitment of macrophages and M2 polarization by endosialin–CD68 interaction. (C) The matrisome plays a key role in hepatocellular carcinoma development by the action of several molecules secreted into the tumor microenvironment, such as MMP, cytokines, integrins, and ADAMs. Abbreviations: aHSCs, activated hepatic stellate cells; qHSCs, quiescent hepatic stellate cells; CAFs, cancer-associated fibroblast cells; CCL2, chemokine (C-C motif) ligand 2; CLCF1, cardiotrophin like cytokine factor 1; ECM, extracellular matrix; EVs, extracellular vesicles; Heps, hepatocytes; HGF, hepatocyte growth factor; HMGB1, high mobility group box protein 1; LSECs, liver sinusoidal endothelial cells; MMP, metalloproteinases; TAMs, tumor-associated macrophages; PDGF, platelet-derived growth factor; TAMs, tumor-associated macrophages; TGF-β, transforming growth factor beta. Created with BioRender.com.