Figure 1.
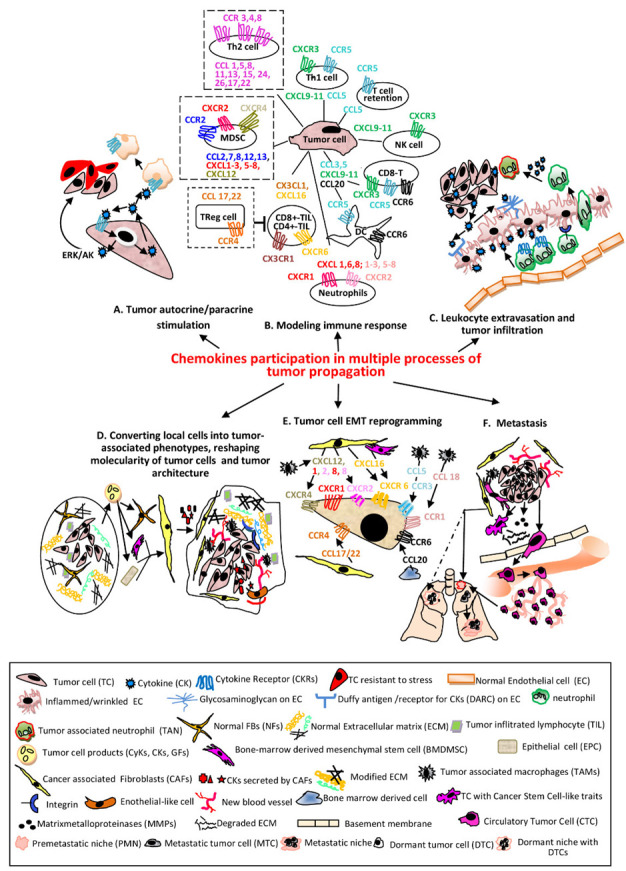
The main processes regulated in various cellular phenotypes by CKs in tandem with their receptors. (A) TCssynthetize and secrete CKs which bind to self CKRs or CKRs expressed by other cells resulting activation of signaling pathways which sustain proliferation, survival and stress-resistance of the same TC (autocrine) or adjacent phenotypes (paracrine) [5]. (B) The CKs secreted by TCs recruit different immune cells in tumor micro environment (TME). The most relevant and documented examples of CKs/CKRs axes are presented. The pro-tumoral associated with poor prognosis are shown with dotted lines (NK—natural killer; Th1/Th2-CD4+T lymphocytes helper type1/type2; CD8+/CD4+-TIL-CD4+/CD8+ Tumor infiltrating lymphocyte; Treg-Regulatory T cell; MDSC—myeloid derived suppressor cell; DC—dendritic cell) [6,7]. (C) CKs secreted by TCs bound to the receptors (glycosaminoglycans and Duffy antigen) of inflamed (wrinkled) ECs are transcytosed and accumulated onto luminal face of ECs; the leucocytes migrate into lumen of the vessel toward CKs and establish firm attachments via overexpressed integrins, traverse endothelium through a gap junction or a pore, follow the extracellular CK gradient and enter the TME becoming TANs [8]. (D) Primary tumors contain NFBs and ECM with an ordered composition adequate to ensure immune infiltrates (left). Secreted factors by TCs convert NFBs and other Stromal Cells (SCs) into CAFs or Tumor Stromal Cells (TSCs-BMDMSCs or EPCs); here for simplicity only CAFs are presented. TSCs release various CKs which increase the number of stationary TAMs, that secrete CKs pro-tumor cell survival, induce angiogenesis, sustain the proliferative, migratory and invasive processes in TCs and vascular mimicry phenomenon, represented by Endothelial-like cells. The advanced tumor ECM composition becomes stiffer, includes modified proteins, stronger interactions via overexpressed integrins, keeping chemotherapeutic drugs and immune cells at tumor periphery (right) [9,10,11]. (E) The process of endothelial to mesenchymal transition (EMT) is essential in enabling TCs for invasion and metastatic dissemination. The most significant CKs secreted by TSCs acting on CKRs expressed on TCs and involved in EMT are presented [12]. (F) The cell signaling pathways activated by CKs in TCs and TSCs define the metastasizing process with the following major events: Inducing Cancer Stem Cell-like traits in TCs (self-renewal, colonies formation, mesenchymal multipotent differentiation) [13]. Remodeling ECM and invasion—the increased MMPs in TME disassemble components of ECM; TCs acquire invadopodia, protrude into the basement membranes, advance and disseminate within surrounding tissues [14]. Intravasation—TCs migrate across the vessel into blood or lymph circulatory systems and become CTCs [15]. Death or survival within circulatory system—until the opportunity of extravasation occurs, CTCs have to overcome hurdles as: lack of GFs/CytKs, the circulatory flow, anoikis, anti-tumor mechanisms of activated NKs/phagocytic cells and form circulating tumor microemboli [16]. Pre-metastatic niches (PMNs) formation—in secondary organs, as result of migration of hematopoietic bone marrow cells and SCs to particular sites in distal organs, with remodeled ECM, occur environmental milieus favorable to later installation and proliferation of TCs. These events facilitate the colonization with TCs, CyKs, GFs and TCs-derived exosomes as well as the suppression of immune system and installation of hypoxia. Distinct PMNs are formed in different organs [17,18]. In addition, dormant niches (DNs) (dotted line) with opposite properties from PMNs are also formed. Extravasation—TCs leave circulatory system and are seeded in PMNs [19]. Dissemination—Unlike DNs which will promote tumor cell dormancy, PMNs will sustain metastatic outgrowth instead. A primary tumor has the ability to seed more than one organ. PMNs are formed in different organs, which control the fate of TCs seeding at each metastatic site. A tumor contains sub-clones which colonize specific organs forming organ-specific metastasis [18].
