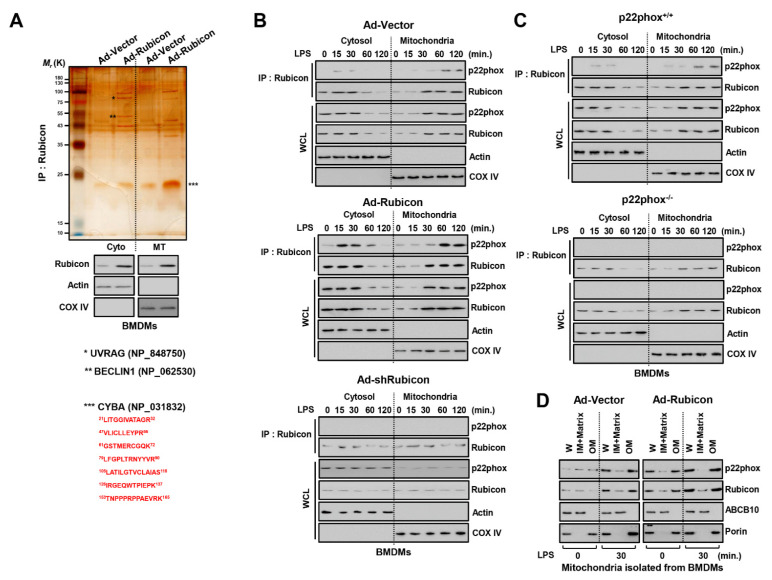
Figure 2.
Rubicon−mediated p22phox translocated to mitochondria. (A) Identification of p22phox as endogenous binding partners of Rubicon in mitochondria. BMDMs were transduced with Ad−Rubicon or Ad-Vector (MOI = 10) for 2 days, followed by nuclear and cytoplasmic fractions separated and subjected to IP with αRubicon. Binding partners were confirmed by silver staining and mass spectrometric analysis (up). BMDMs were subcellularly fractionated and subjected to IB with αRubicon. αTubulin was detected as cytoplasmic protein loading controls. αLamin B1 was detected as nuclear loading controls (middle). The red-colored letters indicate the p22phox (CYBA) peptides identified from mass spectrometry analysis (down). (B,C) BMDMs were transduced with Ad-Rubicon, Ad−shRubicon, or Ad−Vector (MOI = 10) for 2 days (B) or BMDMs from p22phox+/+ and p22phox−/− (C) and stimulated with LPS (100 ng/mL) for indicated times, followed by IP with αRubicon and IB with αp22phox. WCLs were used for IB with αRubicon, αp22phox, αCOX IV, and αActin. (D) Mitochondria isolated from BMDMs were subjected to digitonin extraction to separate the outer-membrane fraction (OM) and the fraction containing the inner membrane and matrix (IM + Matrix). These fractions and whole mitochondria (W) were subjected to IB using αRubicon. Mitochondrial VDAC/porin (an outer membrane protein) and ABCB10 (an inner-membrane protein). Data shown are representative of three independent experiments with similar results.