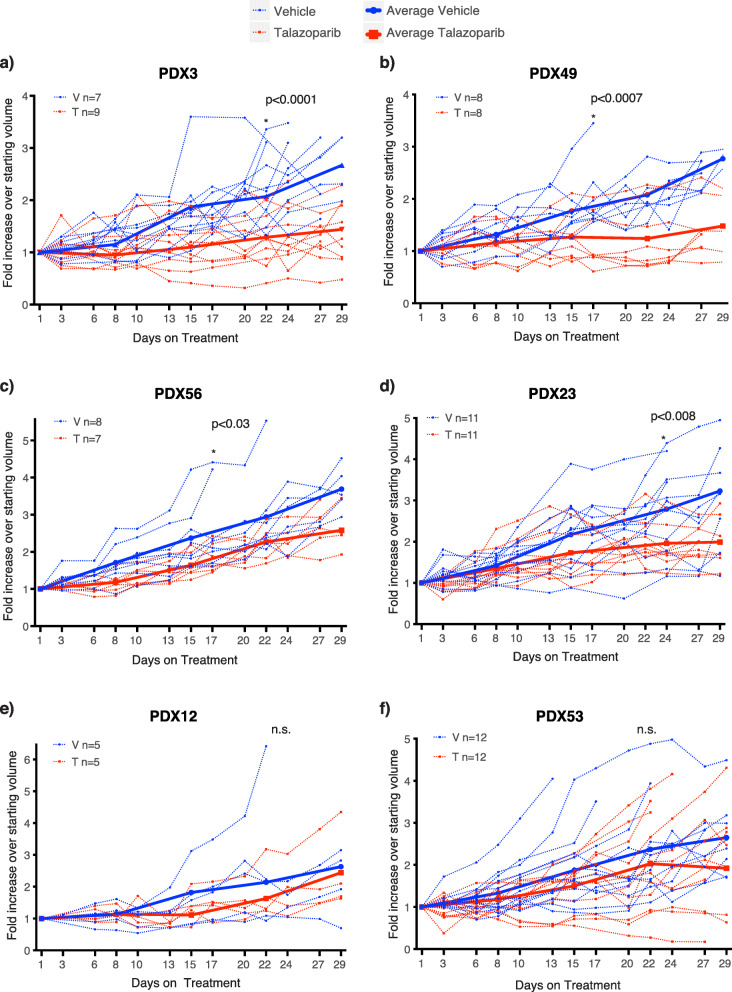
Fig. 5.
Talazoparib responses in EC and UCS PDX models. Talazoparib responses in A PDX03 — CN-high/p53mut UCS; B PDX49 — CN-high/p53mut UCS; C PDX56 — CN-high/p53mut UCS with somatic ARID1A deletion; D PDX23 — CN-high EC; E PDX12 — MMRd EC with somatic PTEN, BRCA2, ATM, and PALB2 mutations; F PDX53 — MMRd EC with somatic PTEN, ATM, BRCA1, and MRE11A mutations. Recipient mice bearing PDX at starting volume of ~150–350 mm3 were randomized to treatment with vehicle or talazoparib (0.33mg/kg) for 28 days (6 days on, one day off) via oral gavage. Analysis for significance between treatment groups was performed using a repeated mixed effects analysis (which can account for random missing measurements) on the day the first mouse was sacrificed based on tumor size (e.g., 17, 22, and 24 days), except for PDX53 where 2 vehicle mice were sacrificed early and excluded. n.s, not significant; *, significant difference (p-value shown)