FIGURE 2. Administration of rCXCL17 does not improve the control of M. tuberculosis HN878 infection in mice.
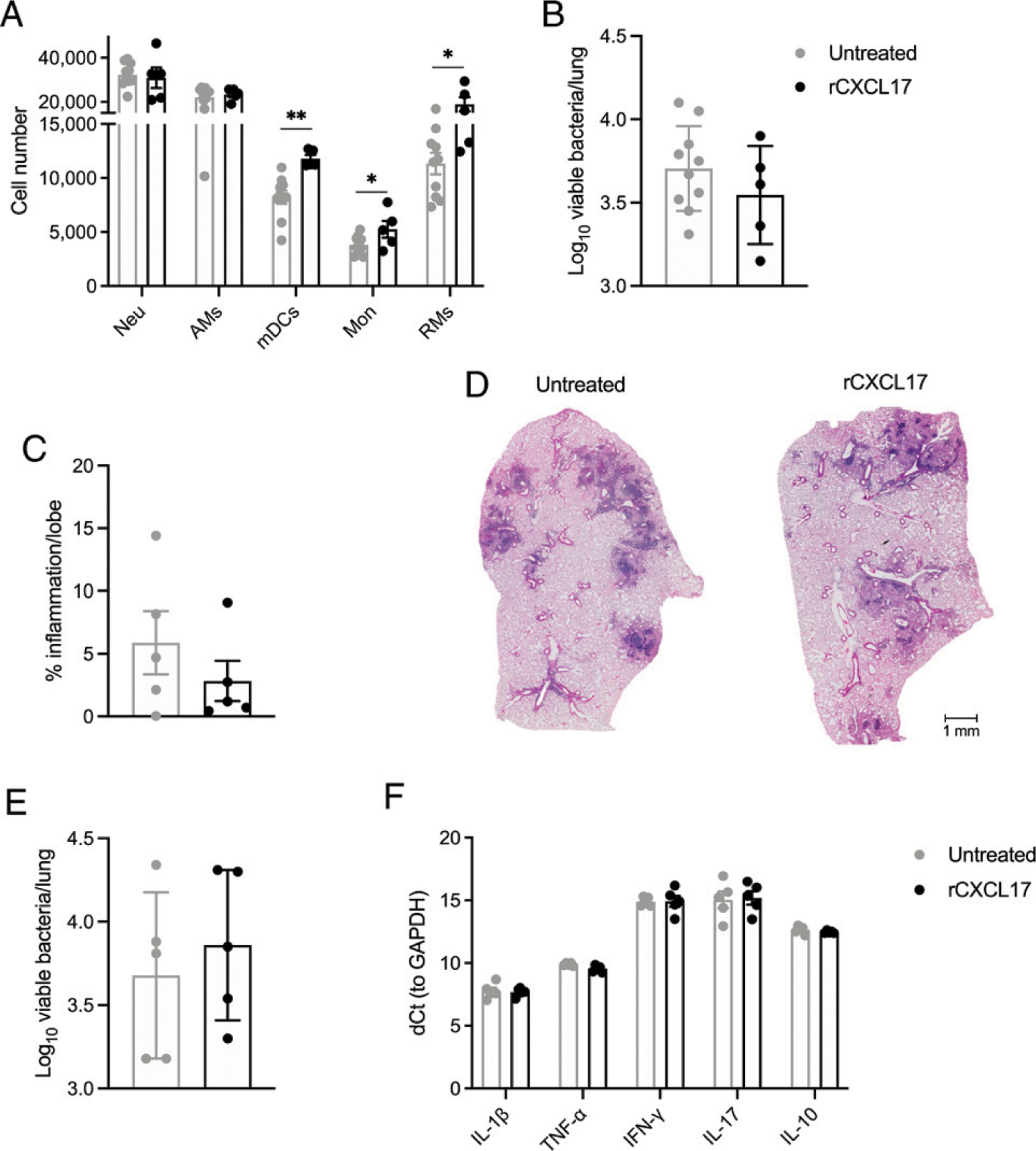
B6 mice were infected with ~100 CFU of aerosolized M. tuberculosis HN878 as before and sacrificed at 30 dpi. Some mice (n = 5) received ~100mg/kg rCXCL17 administered by the intratracheal route at 0, 7, and 14 dpi, whereas a separate group of animals (n = 10) were treated with PBS. (A) Lung myeloid cell populations were enumerated in M. tuberculosis HN878-infected B6 mice treated with rCXCL17 and control animals using flow cytometry. (B) Lung bacterial burden was determined by plating and counting CFUs. (C) Lung area occupied by inflammation was quantified in H&E-stained, formalin-fixed, and paraffin-embedded (FFPE) lungs using NanoZoomer software. (D) Representative microphotographs showing inflammation in H&E-stained FFPE lung sections (original magnification ×10) from rCXCL17-treated and untreated M. tuberculosis HN878-infected B6 mice. (E) Another group of B6 mice infected with M. tuberculosis HN878 was treated with rCXCL17 and sacrificed at 16 dpi. Lung bacterial burden was determined by plating. (F) The expression of several cytokines in the lungs of mice was assessed by real-time PCR. Comparisons between groups were performed using the unpaired Student t test. The data shown represent mean (± SD) values from two to three independent experiments. *p ≤ 0.05, **p ≤ 0.01.
