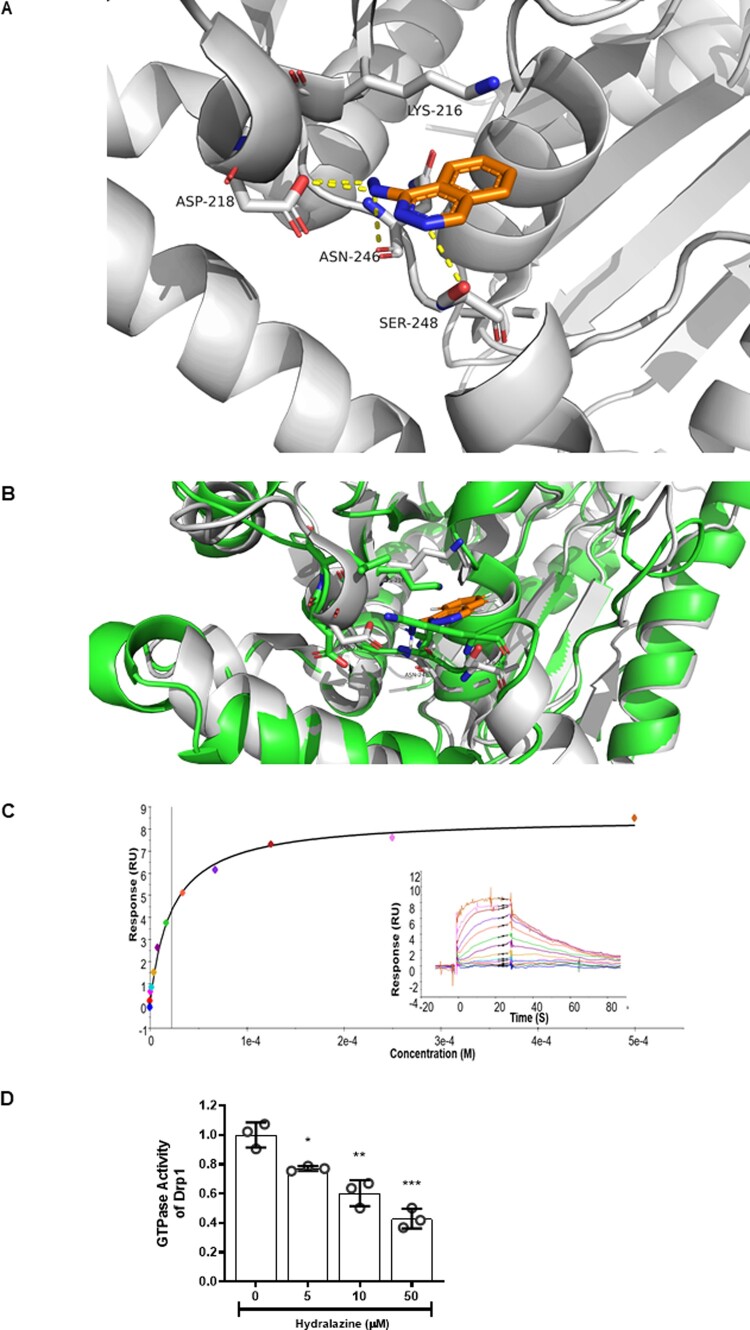
Figure 1.
Hydralazine putatively binds to Drp1 and inhibits its GTPase activity. (A) Molecular docking studies demonstrated that hydralazine (orange structure) putatively binds to the GTPase domain of Drp1 (white structure) via hydrogen bonds (yellow dashed lines) to Asp218, Asn246, and Ser248. Lys216 is able to charge-pi stack to the phthalazine ring of hydralazine, thereby stabilizing its binding to Drp1. (B) Overlay of Drp1 (white) with OPA1 (green) showing that the residues, which we propose are important for the interaction between hydralazine to Drp1 (Lys216, Asn218, Asn246, and Ser248, equivalent to Lys469, Asp470, Thr503, and Lys505 in OPA1) are not present in OPA1. Specifically, the Drp1 Ser248 to OPA1 Lys505 change blocks this binding pocket, thereby preventing hydralazine (orange) from binding to the GTPase domain of OPA1. (C) A representative SPR (Biacore T200) experiment demonstrating a direct dose–response binding interaction between the bound recombinant Drp1 protein exposed to increasing concentrations of hydralazine. Each experiment was run in duplicate. Overall this interaction had a calculated KD of 8.6±1.0 µM. N=3 independent experiments. (D) Hydralazine inhibited GTPase activity of Drp1 in a dose-dependent manner. N=3 independent experiments. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison post-test. Data are expressed as mean±SEM. *P<0.05, **P<0.01, ***P<0.001, vs. control (Drp1 alone).