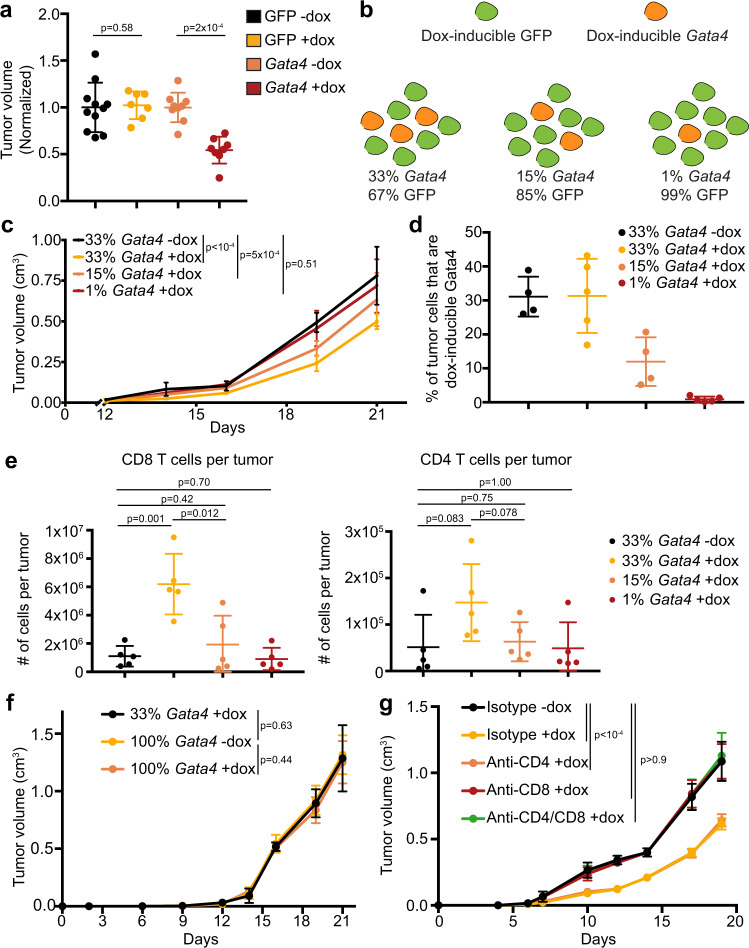
Fig. 4. Gata4 acts in a non-cell-autonomous, CD8 cytotoxic T cell-dependent mechanism to suppress tumor growth.
a Tumor volumes from experiments. GFP+ KP lung cells with either dox-inducible GFP or dox-inducible Gata4 were transplanted subcutaneously into the flanks of C57BL/6J mice. GFP was expressed in all cell types to control for the potential immunogenicity of GFP. Dox administration began the day before tumor cell transplantation. The experiment was performed twice and the combined normalized volumes from both experiments are displayed. Data are mean ± SD and n = 7 tumors for GFP-dox, n = 7 tumors for GFP + dox, n = 9 tumors for Gata4-dox, and n = 8 tumors for Gata4+dox,. P-values are from two-tailed Mann–Whitney tests and n = 11 for GFP − dox, n = 7 for GFP + dox, n = 9 for Gata4 -dox, and n = 8 for Gata4 + dox, where n represents the total number of tumors. b Schematic overview of the experiment to determine whether Gata4 has cell-autonomous or non-cell-autonomous effects on tumor suppression and whether cell cycle regulation is important. KP cells were transduced with pInducer30 Gata4-HA (Thy1.1+ and GFP+). Control KP cells were transduced with pInducer30 GFP-HA (Thy1.1− and GFP+). The cell populations are mixed together at the specified ratios and transplanted into immune-competent C57BL/6J mice. Tumor cells can be flow cytometrically differentiated from non-tumor cells via GFP expression. c Populations outlined in b are mixed with the specified proportions of KP cells with dox-inducible GFP and KP cells with dox-inducible Gata4. Cells were transplanted into the flanks of immune-competent C57BL/6J mice; dox was started in the indicated groups one day before tumor cell transplant and administered continuously until endpoint. Tumor volumes throughout the course of the experiment are shown. Data are mean ± SD. P-values are from two-tailed Mann–Whitney test at the day 19 timepoint and n = 10 mice per group. d Flow cytometry-based quantification of the proportion of dox-inducible Gata4 tumor cells (GFP+Thy1.1+) as a fraction of all tumor cells (GFP+) at the experimental endpoint of the experiment shown in c. Data are mean ± SD and n = 4 or 5 tumors per group. e Flow cytometry-based immunoprofiling of TILs in tumors from the experiment shown in c. Tumors were extracted at the experimental endpoint, dissociated to a single-cell suspension, and stained for the indicated TIL subtype. Data shown are quantification of the number of cells of the indicated TIL subtype per tumor. P-values are from unpaired two-way t-tests and n = 5 per group. f KP cells with dox-inducible Gata4 at the specified ratios were transplanted into Rag1−/− C57BL/6J mice. The 33% Gata4 population was composed of 33% of cells with dox-inducible Gata4 and 67% of cells with dox-inducible GFP as in c. Dox was administered to mice one day before tumor cell transplant and was administered continuously until endpoint. Tumor volumes are shown. Data shown are mean ± SD and n = 10 per group combined from 2 independent experiments. P-values are from a two-tailed Mann–Whitney test at the Day 21 timepoint. g C57BL/6J mice received depleting antibodies against CD4+, CD8+, CD4+ and CD8+ T cells, or an isotype control antibody. All mice received the same total amount of antibody. The single depletion groups received isotype control antibody in addition to depletion antibody in order to ensure that all groups of mice received the same amount of antibody as the double depletion group. KP cells with dox-inducible Gata4 were transplanted into the flanks of the pre-depleted mice. Dox was initiated one day before transplant in the indicated groups and was administered continuously until the experimental endpoint. Tumor volumes are shown. Data are mean ± SD and n = 10 per group combined from 2 independent experiments. P-values are from two-tailed Mann–Whitney tests at Day 19 timepoint. Specifically, p-values are as follows: p < 0.0001 for isotype −dox versus isotype +dox, p < 0.0001 for isotype -dox versus anti-CD4 + dox, p = 0.9243 for isotype −dox versus anti-CD8 + dox, and p = 0.9425 for isotype −dox versus anti-CD4/CD8 + dox. For this figure, source data are provided as a Source Data file.