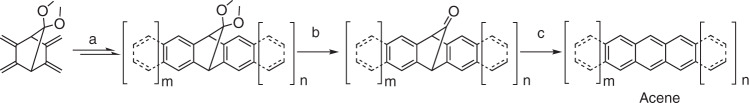
Fig. 2. Schematic strategy of the preparation of various acenes starting from 7,7‐dimethoxy‐2,3,5,6‐tetramethylenebicyclo[2.2.1]-heptane.
Diels-Alder addition (a) with arynes, followed by aromatization gives a non-planar bridging dimethylketal, which can be deprotected (b) to yield a polyaromatic precursor bridged by a carbonyl group. (c) Solid-state thermal or photochemical decarbonylation gives the acene with only carbon monoxide as by-product.