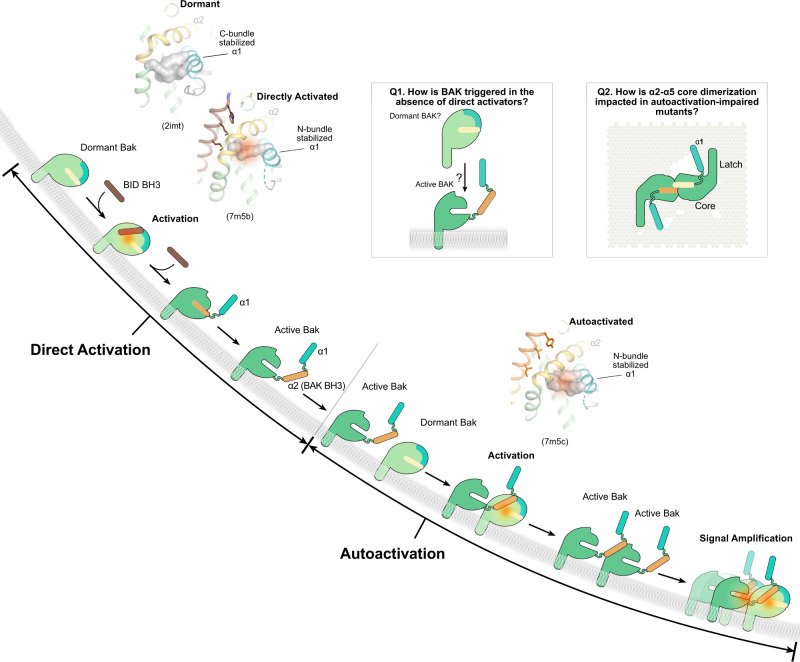
Fig. 7. Cooperation of autoactivation and direct activation in apoptosis initiation by BAK.
Direct BAK activation by BH3-only proteins cooperates with BAK autoactivation in trans to lower BAK threshold and to amplify the response leading to mitochondrial poration (Supplementary Movie 2). BAK autoactivation involves binding the exposed BH3 of active BAK to the activation groove of dormant BAK. Our structural analyses of autoactivated (7m5c) and directly activated (7m5b) BAK reveal BH3 ligand-induced conformational changes in the protein core that promote helix α1 destabilization as the mechanistic basis of BAK activation (inset cartoons). In activated BAK complexes helix α1 electrostatic network is destabilized as it rearranges within the N-bundle (helices α1-α2), which dissociates in the presence of membranes but not in solution. In contrast, in dormant, apo BAK (2ims) and an inactivated BAK complex bound to a rationally-designed BID-like BH3 peptide (7m5a), helix α1 is stabilized by the electrostatic network within the C-bundle (helices α3-α8), which prevents N-bundle dissociation at membranes (Supplementary Movie 1). How active BAK porates membranes is unclear but oligomerization of the BAK α2-α5 core dimers has been postulated. Mechanistic questions not addressed in our study are inset.