Abstract
There are a great number of beneficial commensal microorganisms constitutively colonizing the mucosal lining of the lungs. Alterations in the microbiota profile have been associated with several respiratory diseases such as pneumonia and allergies. Lung microbiota dysbiosis might play an important role in the pathogenic mechanisms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as well as elicit other opportunistic infections associated with coronavirus disease 2019 (COVID-19). With its increasing prevalence and morbidity, SARS-CoV-2 infection in pregnant mothers is inevitable. Recent evidence shows that angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) act as an entry receptor and viral spike priming protein, respectively, for SARS-CoV-2 infection. These receptor proteins are highly expressed in the maternal-fetal interface, including the placental trophoblast, suggesting the possibility of maternal–fetal transmission. In this review, we discuss the role of lung microbiota dysbiosis in respiratory diseases, with an emphasis on COVID-19 and the possible implications of SARS-CoV-2 infection on pregnancy outcome and neonatal health.
Keywords: COVID-19, microbiome, microbiota dysbiosis, pregnancy outcome, SARS-CoV-2
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a betacoronavirus that is known to infect humans and other mammals such as bats. Its zoonotic nature is well proven. 1 The outbreak of the dreadful coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, was first reported in December 2019 in Wuhan, China. 2 The outbreak currently ravaging the world was declared a pandemic on 11 March 2020 by the World Health Organization (WHO). 3 The pandemic has caused a devastating global health and economic burden, 4 with over 3 million deaths as of 31 May 2021. 5 COVID-19 is a complex multisystemic disease, and the spectrum of manifestations can vary from asymptomatic disease to severe acute respiratory distress syndrome, renal dysfunction, hyperimmune state, and sepsis, eventually leading to death.2,6–8 The pathogenesis of COVID-19 is not fully understood, and its complexity has evolved to the point where microbiota dysbiosis could be implicated in the pathological process.9–12
The human microbiome refers to the entire microbial composition of an organ or system, including the microorganisms, their surrounding environmental conditions, genomes, and host interactions. 13 Microbiota is the community of microorganisms colonizing the human body. The human microbiota plays a role in nutrient metabolism, fat store regulation, 14 modulation of the immune response,8,15 and maintaining host homeostasis. 16 The interplay between commensal microbiota and host immunity is important in maintaining mutualism and homeostasis, and perturbation of this interaction could lead to disease.16,17
Microbiota dysbiosis has been associated with several respiratory diseases, such as chronic obstructive pulmonary disease,18,19 asthma,20,21 cystic fibrosis,22,23 tuberculosis, 24 and lung cancer. 25 The nasopharynx is the primary site for pathogen colonization, a mechanism that contributes to the onset of respiratory diseases; any imbalance in the mucosal nasopharyngeal microbiota may play a vital role in susceptibility to viral respiratory infections.26,27 Studies on microbiota composition in a healthy person and patients with respiratory disease suggest that microbiota may play a crucial role in the genesis and clinical development of the disease, 26 including bronchiolitis in infants, 28 and asthma. 29 Edouard et al. reported that the microbiota from a healthy nasopharyngeal swab was composed of aero-anaerobic bacteria and was altered during a viral respiratory infection. 26 COVID-19 patients are said to show significant alteration in the gut microbiota during the period of hospitalization and at an all-time point during intensive care.9,10 Similarly, Man et al. recently showed a strong correlation between viral and bacterial microbiota in the upper respiratory tract and the severity and presence of childhood respiratory infection later in life. 27 Thus, alteration of the microbiota profile during SARS-CoV-2 infection might be associated with disease severity.
Emerging and recent studies have shown that lung microbiota dysbiosis could be associated with pulmonary diseases such as pulmonary fibrosis, 30 and this may impact the outcome of COVID-19 cases.31–33 The imbalance between gut and lung microbiota might compromise host immune response during episodes of SARS-CoV-2 infection, leading to uncontrolled inflammation. 34 However, it is still unclear whether microbiota dysbiosis contributes to the inflammation or whether this is entirely the effect of COVID-19. The focus of this review is twofold. Firstly, to understand the role of lung microbiota dysbiosis on the pathogenesis of respiratory diseases with emphasis on the maternal–fetal transmission of COVID-19, and secondly, to elucidate the impact of SARS-CoV-2 infection on pregnancy, and maternal and child health.
Lung microbiota in respiratory diseases
Respiratory microbiota are often referred to as the gate-keepers to respiratory health, and their involvement in maintaining lung immunity and homeostasis has been studied widely . 35 During the first few weeks of life, distinct lung microbiota exist. 36 For example, the Staphylococcus aureus population in the nasopharyngeal microbiota niche declines gradually, and there is a simultaneous increase in potential beneficial commensals such as Dolosigranulum pigrum and Corynebacterium species. 37 However, a decline in beneficial bacteria has been associated with the risk of pneumonia in children. 38 Bosch et al. reported that children delivered by caesarean section were more likely to have delayed development of nasopharyngeal commensals Dolosigranulum and Corynebacterium profiles early in life, and this might influence their respiratory health later in life. 37 Abundance of nasopharyngeal Dolosigranulum (especially Dolosi-granulum pigrum) and Corynebacterium is an indication of a healthy respiratory microbiome. 39 Dysbiosis in this diverse microbiota profile was associated with several respiratory pathologies (Table 1).40–51 Dolosigranulum is a rare opportunistic pathogen that has been confirmed to cause different types of septicemia and pneumonia, while Corynebacterium is abundant in children free of Streptococcus pneumoniae. 50 Lack of airway Corynebacterium might be associated with post-influenza pneumonia.50,52 The consequences of lung microbiota dysbiosis contribute to the worst pathological outcomes during respiratory viral infection.53,54 This is further discussed with emphasis on COVID-19.
Table 1.
The association of lung microbiota with respiratory diseases.
| Model (species) | Respiratory disease | Major findings | References |
|---|---|---|---|
| Human | COPD | Increased Firmicutes Proteobacteria Actinobacteria phyla, and Streptococcus, Corynebacterium, Haemophilus, Pseudomonas, Rothia, Moraxella, Lactobacillus genus in COPD lung microbiota | Erb-Downward et al. 40 , Huang et al. 41 , Pragman et al. 42 , Pragman et al. 43 , Sze et al. 44 |
| Human | Asthma | Increased eosinophils correlates positively with abundant Actinobacteria (Streptomyces and Propionicimonas) | Huang et al. 45 |
| Human | Allergic rhinitis | High IgE titer correlate with low nasal microbial biodiversity with relatively high Staphylococcus aureus and decreased Propionibacterium acnes | Hyun et al. 46 |
| Mice | Allergy | Commensal bacteria-derived signal limit lung allergic inflammation | Hill et al. 47 |
| Human | Rhinitis | Haemophilus, Neisseria, and Moraxella increased significantly in children with rhinitis. Moraxella spp. is associated with children with Rhinitis interacting mite sensitization. Airway Leptotrichia is found in amount in mite-sensitized asthma in children | Chiu et al.48,49 |
| Human | Allergic rhinitis | Airway Haemophilus spp. are positively correlated with IgE levels | Chiu et al. 49 |
| Human | Influenza | Enriched Staphylococcus and Dolosigranulum taxa in the nasopharyngeal region | Ding et al. 50 |
| Human | Acute respiratory tract infection (RSV or Rhinovirus) | Abundant airway Haemophilus in RSV-positive infants | Rosas-Salazar et al. 51 |
COPD, Chronic obstructive pulmonary disease.
Lung microbiota dysbiosis in COVID-19 patients
Few studies have examined the role of lung microbiota in mild and severe COVID-19 patients.31,55–57 A recent study on the analysis of lung microbiota from 20 deceased patients who had severe COVID-19 revealed that the lung microbiota was enriched with bacteria and fungi genera Acinetobacter and Cutaneotrichosporon, respectively, 56 and that fatal COVID-19 episodes might be associated with complex microbial superinfection such as invasive pulmonary aspergillosis. 58 In addition, gut Enterobacteriaceae were also found to predominate in the lungs of deceased COVID-19 patients. 56 This finding might suggest the possibility of intestinal to respiratory microbiome crosstalk or migration. While the mechanism by which this could occur remains elusive, 59 it is plausible that endotoxin secreted by pathogenic Enterobacteriaceae affect gut and lung epithelial cells, thereby leading to heightened pulmonary inflammation. 56 De Maio et al. reported that patients with mild COVID-19 showed no statistical difference in their nasopharyngeal bacterial profile compared with non-infected patients. 60 As microbiota dysbiosis occurs in severe COVID-19 patients, it is not surprising that disturbance in lung microbiota might play a role in SARS-CoV-2 pathogenesis.
Pathogenesis of SARS-CoV-2 infection
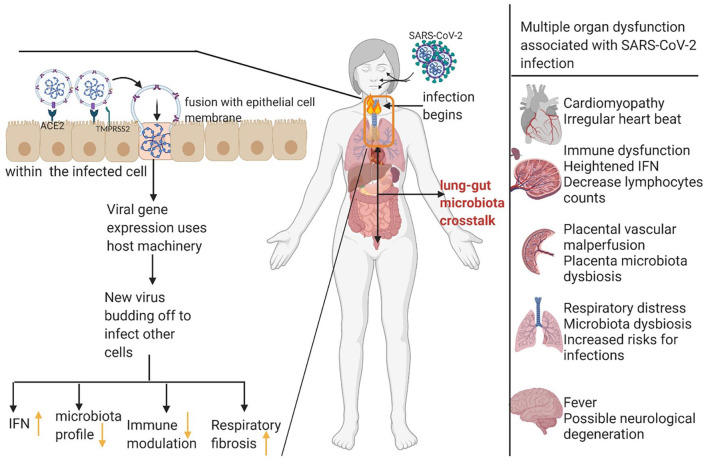
Research on the pathogenesis of SARS-CoV-2 infection in humans is still evolving. The use of animal models is vital for understanding SARS-CoV-2 pathogenesis. 61 Using transgenic mice expressing human angiotensin-converting enzyme 2 (ACE 2), Jiang et al. reported that SARS-CoV-2 viral particles were found in the lung and brain region. 61 They also found that, between the fourth and seventh day of infection, there was significant body weight loss, host immune and cardiac dysfunction, respiratory distress, and even death. 61 Likewise, Israelow et al. reported heightened inflammatory interferon signatures in the lungs similar to COVID-19 patients. 62 Mechanistically, SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2), and it also utilizes transmembrane protease serine 2 (TMPRSS), both expressed on peripheral tissues, including lungs, to gain entry into the host,63–65 thereby affecting host physiological functions (Figure 1). Following this, SARS-CoV-2 can co-infect multiple organs apart from the lungs. The full understanding of human SARS-CoV-2 pathogenesis is currently incomplete and is a rapidly developing area of science.66,67
Figure 1.
Representation of possible pathogenesis of SARS-CoV-2 and effects on mammalian organs. SARS-CoV-2 binds to ACE2, and protease activity of TMPRSS2 facilitates entry into the lung epithelial cells. The pathogenesis of SARS-CoV-2 induces heightened inflammation, microbiota dysbiosis, and possible adverse outcomes such as respiratory distress, and cardiomyopathy, thus complicating host health. (Figure created in Biorender).
ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease serine 2.
SARS-CoV-2 receptors and target cells
ACE2 is the main entry receptor for SARS-CoV-2. The virus utilizes TMPRSS2 as viral spike (S) protein priming. 64 These receptor proteins are expressed predominantly in pulmonary and extrapulmonary organs, including lung type II pneumocytes, nasal goblet cells, placenta, 65 ileal absorptive enterocytes, 68 intestine, 69 liver, and heart. 63 They are also expressed widely in specific cell types, such as decidual stromal cells, placental cytotrophoblasts, and syncytiotrophoblasts at the maternal-fetal interface.70,71 ACE2 is a carboxypeptidase that cleaves angiotensinogen into smaller angiotensin peptides. Early pathogenesis of COVID-19 requires the attachment of the viral S protein to epithelial ACE2, thereby inducing endocytosis, which is followed by priming the viral S protein by TMPRSS2 protease activity, facilitating SARS-CoV-2 entry into the host cell. 63 Apart from ACE2 and TMPRSS2, only a few other proteins, including transducin-like enhancer protein 3 (TLE3) and lysyl oxidase (LOX), which interacts with SARS-CoV-2, were found in an in silico analysis to be upregulated in early and term placental tissue, respectively. 65 LOX is expressed highly in both human fetal membranes and mesenchymal cells of the placenta.65,72 LOX family proteins are extracellular enzymes that participate in reproduction, and an altered expression of LOX protein is associated with endometriosis, 73 impaired placental trophoblast migration, and preeclampsia. 74 SARS-CoV-2 interacts with proteins that are involved in placental function, implantation, and successful decidualization, thus implying a probable route of fetal infection.
Possible maternal–fetal transmission route
The transmission of SARS-CoV-2 from a pregnant mother to a developing fetus is quite rare but possible. 70 With increasing research on the COVID-19 pandemic, more findings suggest that vertical transmission of SARS-CoV-2 is possible. 75 For instance, ACE2 and TMPRSS2 are expressed at the maternal–fetal interface, which indicates the possibility of in utero transmission.76,77 Similarly, Fenizia et al. reported that SARS-CoV-2 genome was found in the umbilical cord blood, at-term placentas, breast milk, and vaginal mucosa in 1 out of 31 pregnant mothers involved in their study. 70 Babies born to mothers who tested positive for COVID-19 have a detectable amount of the virus-specific antibodies in their sera. 78 Vivanti et al. recently reported possible transmission of SARS-CoV-2 between pregnant mothers and their developing fetuses using comprehensive immunological and virological techniques. 79 All samples collected, including amniotic fluid, were positive for SARS-CoV-2, and the developing fetuses showed irregular fetal heartbeats, 79 accompanied by neurological defects such as encephalitic symptoms early in life. 80 In a longitudinal study, mothers who tested positive for COVID-19 showed a positive serological test for IgM in breast milk between 3 and 68 days after the onset of COVID-19 symptoms. 81 Consecutively, others reported IgM at day 8 and IgG on day 28 in the breast milk of nursing infected mothers, cord blood, and neonatal serum. 82 However, there was no detectable trace of SARS-CoV-2 found in breast milk. 82 At the moment, the transmission of SARS-CoV-2 via breast milk remains inconclusive.70,78 It was suggested that proper hygiene during breastfeeding might contribute to a low risk of transmitting SARS-CoV-2 in neonates. 83
Microbiota dysbiosis during pregnancy and birth
Pregnancy is a physiological state that involves changes in hormonal homeostasis, immunity, metabolic processes, and microbiota composition in order to support fetal growth and development. 84 Until recently, there was speculation that developing fetuses are germ-free since the womb and placenta are sterile. 84 However, the uterus is, after all, not sterile but home to most beneficial commensals, suggesting that fetuses are exposed to commensals during development, and this, in turn, might influence fetal immune development. 85 For the first time, Al Alam et al. reported possible traces of human fetal and placenta microbiome as early as the first trimester in pregnancy, and these microbiotas inhabit the fetal lung. 86 The placenta has also been shown to consist of a diverse microbiota profile. 87 Using genomic DNA sequencing, Parnell et al. reported that distinct microbiota diversities exist in the placenta. 87 Their findings also showed that Ralstonia insidiosa and Mesorhizobium spp. were abundant in both the placental villi and basal plate while the Lactobacillus spp. were abundant in the fetal amniotic membrane. The composition of microbiota early and later in life of these neonates might depend on the mode of birth (either via vaginal delivery or caesarean section) and maternal nutrition.88–90 We postulate that distinct fetal lung microbiota might be established in the early phases of pregnancy.
The importance of microbiota in health has been well established. 91 Lactobacilli and Prevotella dominate the vaginal microbiota,92,93 and Proteobacteria and Actinobacteria are abundant in gut microbiota. In contrast, Actinobacteria, Fermicutes, and Bacteroides dominate oral microbiota. 94 Disturbance in the oral, intestinal, placenta, and vaginal microbiota during pregnancy either as a result of infection or stress such as hormonal imbalance might lead to complications for the baby, including metabolic programming during development, 95 preterm birth, 96 and altered neurological development.94,97
Potential implications of COVID-19 in pregnancy
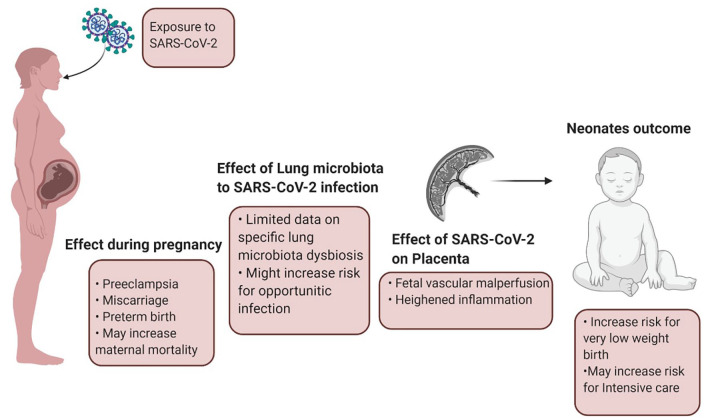
It is possible that COVID-19 might have an adverse impact on pregnant mothers and their babies, but the exact mechanism is still unknown.98,99 SARS-CoV-2 infection during pregnancy might affect placental morphology, thereby impacting the pregnancy adversely (Figure 2). The placenta is a unified organ responsible for mother to fetus nutrient transport, and it accommodates numerous commensal microorganisms. Placental microbiota dysbiosis during pregnancy might lead to undesired outcomes.100–102 There is increasing evidence that SARS-CoV-2 is detected in placental tissue and amniotic fluid in preterm fetuses born to SARS-CoV-2 infected mothers.103,104 Histological analysis of placental morphology in neonates born to SARS-CoV-2 infected mothers revealed fetal vascular malperfusion, 105 maternal vascular malperfusion, 106 heightened macrophage, and lymphocytes infiltration. 103 Heightened placental inflammation might result in early-onset of preeclampsia, poor maternal condition, and adverse birth outcomes. 103
Figure 2.
Representation of exposure to SARS-CoV-2 infection during pregnancy and neonate/fetus outcome. SARS-CoV-2 infection in pregnancy may induce vascular malperfusion in the placenta. This might alters fetal growth and development, which may cause negative pregnancy outcome, as well as have adverse effects in neonates health later in life. (Figure created in Biorender).
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
There are reports that describe the outcomes of neonates born to mothers with SARS-CoV-2. 107 COVID-19 affects successful fetal development and might cause preeclampsia, miscarriages, fetal growth restriction, and preterm birth.107,108 More pregnant women with COVID-19 are now opting for caesarean section during the third trimester because of the uncertainty of mother-child transmission via vaginal delivery.107,109 However, babies delivered by a caesarean section might lose out on inheriting most of the beneficial vaginal microbiome. Pregnant women are in an immunosuppressed state and recent studies show that they are susceptible to respiratory diseases such as severe pneumonia. 109 When infected with SARS-CoV-2, they present with clinical symptoms such as fever, cough, 83 myalgia, and rashes 75 and laboratory findings show elevated C-reactive protein and lymphocytopenia. 110
Emerging research has also shown that SARS-CoV-2 infection during pregnancy might cause miscarriages, 111 small for gestational age, low birthweight (<2500 g), preterm births, 70 and even fetal death.112,113 Of the 125 pregnant women admitted to an intensive care unit in a multicenter unit in Turkey, about 86.5% of neonates born to such mothers were isolated in the neonatal intensive care unit for respiratory support. 114 Symptoms associated with neonates born to SARS-CoV-2 infected mothers include respiratory distress, gastrointestinal disturbance, fever, irregular heart rate, abnormal liver function, poor immune function, multiple organ failure, 115 unexplained rashes, and facial ulceration. 116 Data from a large cohort (n = 91,412) of women of reproductive age in the United States (US) with laboratory-confirmed COVID-19 revealed that the incidence of the disease might be higher in pregnant women (31.5%) than non-pregnant women (5.8 %), 117 and there was a possibility that these figures might continue to rise. In another smaller cohort study involving 46 patients, it was found that COVID-19 patients with underlying medical conditions such as obesity had an increased risk for pregnancy complications. 118 With the emerging findings on possible maternal–fetal transmission and pregnancy outcome, more research is warranted on the effects of SARS-CoV-2 infection during pregnancy and neonatal health.
Conclusion and future direction
Lung microbiota dysbiosis is associated with respiratory distress and increases the risk of respiratory infection. SARS-CoV-2 infection during the gestational period affects maternal health and may cause severe complications for the developing fetus, such as metabolic programming and restricted growth leading to preterm birth. With the emerging findings on possible maternal–fetal transmission and adverse pregnancy outcomes in patients infected with SARS-CoV-2, we postulate that lung-gut microbiota crosstalk exists and maternal–fetal microbiota exchange during pregnancy and birth may be a signature for neonatal, infant, and child health. However, it is premature to speculate on the effect of SARS-CoV-2-driven lung microbiota dysbiosis and its eventual impact on pregnancy outcomes due to maternal infection. Future larger scale, longitudinal, population studies should further explore: (1) the mechanisms whereby SARS-CoV-2 influences specific maternal lung microbiota during pregnancy, (2) whether such specific dysbiosis is transferred during pregnancy to the developing fetus, and (3) whether there is a correlation between specific fetal microbiota profiles at different trimesters with pregnancy outcome and neonatal health. Results of these emerging studies might be useful in providing guidelines for managing SARS-CoV-2 infection during pregnancy.
Acknowledgments
The authors thank the two anonymous reviewers for their suggestions and comments on an earlier version of this paper. We are also grateful to Tara Pylate for proofreading the final version.
Footnotes
Author contributions: Conceptualization, HCE; methodology, HCE; validation, HCE, KTA, GRO, OAA; investigation, HCE, CAD, IJE, MJA, JAG, KTA, GRO, OAA; resources, HCE, OAA; data curation, HCE; writing—original draft preparation, HCE, CAD, IJE, MJA, JAG; writing—review and editing, HCE, CAD, IJE, MJA, JAG, KTA, GRO, OAA; visualization, HCE; supervision, OAA. All authors have read and agreed to the published version of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Oyelola A. Adegboye  https://orcid.org/0000-0002-9793-8024
https://orcid.org/0000-0002-9793-8024
Contributor Information
Henry C. Ezechukwu, Department of Medical Biochemistry, Eko University of Medicine and Health Sciences, Ijanikin, Lagos, Nigeria
Cornelius A. Diya, Department of Medical Biochemistry, Eko University of Medicine and Health Sciences, Ijanikin, Lagos State, Nigeria
Ifunanya J. Egoh, Department of Virology, University of Ibadan, Ibadan, Nigeria
Mayowa J. Abiodun, Department of Cell Biology, University of Lagos, Akoka, Lagos State, Nigeria
John-Ugwuanya A. Grace, Medbury Medical Service, Ikeja, Lagos State, Nigeria
God’spower R. Okoh, College of Public Health, Medical and Veterinary Sciences, Division of Tropical Health and Medicine, James Cook University, Townsville, QLD, Australia
Kayode T. Adu, ProbioWorld Consulting Group, James Cook University, Townsville, QLD, Australia Cann Group Ltd., Walter and Eliza Hall Institute, VIC, Australia.
Oyelola A. Adegboye, Australian Institute of Tropical Health and Medicine, James Cook University, Townsville, QLD 4811, Australia; College of Public Health, Medical and Veterinary Sciences, Division of Tropical Health and Medicine, James Cook University, Townsville, QLD, Australia; World Health Organization Collaborating Center for Vector-Borne and Neglected Tropical Diseases, College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, QLD, Australia.
References
- 1. Wacharapluesadee S, Tan CW, Maneeorn P, et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat Commun 2021; 12: 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol 2020; 11: 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (2020, accessed 10 December 2020).
- 4. Pak A, Adegboye OA, Adekunle AI, et al. Economic consequences of the COVID-19 outbreak: the need for epidemic preparedness. Front Public Health 2020; 8: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Coronavirus (COVID-19) Dashboard [Internet], https://covid19.who.int (accessed 2 June 2021).
- 6. Micarelli D, Moccia F, Costantini S, et al. COVID-19 is a complex disease with wide spectrum of clinical patterns and an emerging problem for nephrologist. J Nephropathol 2020; 9: e33. [Google Scholar]
- 7. Miesbach W, Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost 2020; 26: 1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020; 75: 1564–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zuo T, Zhang F, Lui GCY, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020; 159: 944–955.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuo T, Zhan H, Zhang F, et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology 2020; 159: 1302–1310.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khatiwada S, Subedi A. Lung microbiome and coronavirus disease 2019 (COVID-19): possible link and implications. Hum Microb J 2020; 17: 100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu S, Chen Y, Wu Z, et al. Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza. Clin Infect Dis 2020; 71: 2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chotirmall SH, Gellatly SL, Budden KF, et al. Microbiomes in respiratory health and disease: an Asia-Pacific perspective. Respirology 2017; 22: 240–250. [DOI] [PubMed] [Google Scholar]
- 14. Rivera-Piza A, Lee S-J. Effects of dietary fibers and prebiotics in adiposity regulation via modulation of gut microbiota. Appl Biol Chem 2020; 63: 2. [Google Scholar]
- 15. Belkaid Y, Hand T. Role of the microbiota in immunity and inflammation. Cell 2014; 157: 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res 2020; 30: 492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levin BR, Antia R. Why we don’t get sick: the within-host population dynamics of bacterial infections. Science 2001; 292: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 18. Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J 2016; 47: 1082–1092. [DOI] [PubMed] [Google Scholar]
- 19. Beech AS, Lea S, Kolsum U, et al. Bacteria and sputum inflammatory cell counts; a COPD cohort analysis. Respir Res 2020; 21: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arrieta M-C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015; 7: 307ra152. [DOI] [PubMed] [Google Scholar]
- 21. Simonyte Sjödin K, Vidman L, Rydén P, et al. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Curr Opin Allergy Clin Immunol 2016; 16: 390–395. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen S, Needham B, Leach ST, et al. Disrupted progression of the intestinal microbiota with age in children with cystic fibrosis. Sci Rep 2016; 6: 24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burke DG, Fouhy F, Harrison MJ, et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol 2017; 17: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maji A, Misra R, Dhakan DB, et al. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ Microbiol 2018; 20: 402–419. [DOI] [PubMed] [Google Scholar]
- 25. Vengoechea JJ, Ponce-Alonso M, Figueredo AL, et al. Changes in the pulmonary microbiome associated with lung cancer. Eur Respir J 2019; 54(Suppl. 63): PA3663. [Google Scholar]
- 26. Edouard S, Million M, Bachar D, et al. The nasopharyngeal microbiota in patients with viral respiratory tract infections is enriched in bacterial pathogens. Eur J Clin Microbiol Infect Dis 2018; 37: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 27. Man WH, van Houten MA, Mérelle ME, et al. Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case-control study. Lancet Respir Med 2019; 7: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasegawa K, Linnemann RW, Mansbach JM, et al. The fecal microbiota profile and bronchiolitis in infants. Pediatrics 2016; 138: e20160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frati F, Salvatori C, Incorvaia C, et al. The role of the microbiome in asthma: the gut–lung axis. Int J Mol Sci 2018; 20: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Dwyer DN, Ashley SL, Gurczynski SJ, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199: 1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aktas B, Aslim B. Gut-lung axis and dysbiosis in COVID-19. Turk J Biol 2020; 44: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Din AU, Mazhar M, Waseem M, et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother 2021; 133: 110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han Y, Jia Z, Shi J, et al. The active lung microbiota landscape of COVID-19 patients. medRxiv, 2020. DOI: 10.1101/2020.08.20.20144014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahlawat S, Sharma KK. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res 2020; 286: 198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Man WH, de Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 2017; 15: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biesbroek G, Tsivtsivadze E, Sanders EAM, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 2014; 190: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 37. Bosch AATM, Levin E, van Houten MA, et al. Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine 2016; 9: 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brugger SD, Eslami SM, Pettigrew MM, et al. Dolosigranulum pigrum cooperation and competition in human nasal microbiota. mSphere 2020; 5: e00852-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lappan R, Imbrogno K, Sikazwe C, et al. A microbiome case-control study of recurrent acute otitis media identified potentially protective bacterial genera. BMC Microbiol 2018; 18: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 2011; 6: e16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang YJ, Sethi S, Murphy T, et al. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 2014; 52: 2813–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pragman AA, Kim HB, Reilly CS, et al. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One 2012; 7: e47305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pragman AA, Lyu T, Baller JA, et al. The lung tissue microbiota of mild and moderate chronic obstructive pulmonary disease. Microbiome 2018; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in severe asthma: associations with disease features and severity. J Allergy Clin Immunol 2015; 136: 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hyun D-W, Min HJ, Kim M-S, et al. Dysbiosis of inferior turbinate microbiota is associated with high total IgE levels in patients with allergic rhinitis. Infect Immun 2018; 86: e00934-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hill DA, Siracusa MC, Abt MC, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med 2012; 18: 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chiu C-Y, Chan Y, Tsai Y-S, et al. Airway microbial diversity is inversely associated with mite-sensitized rhinitis and asthma in early childhood. Sci Rep 2017; 7: 1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chiu C-Y, Chan Y, Tsai M-H, et al. Cross-talk between airway and gut microbiome links to IgE responses to house dust mites in childhood airway allergies. Sci Rep 2020; 10: 13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ding T, Song T, Zhou B, et al. Microbial composition of the human nasopharynx varies according to influenza virus type and vaccination status. mBio 2019; 10: e01296-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rosas-Salazar C, Shilts MH, Tovchigrechko A, et al. Differences in the nasopharyngeal microbiome during acute respiratory tract infection with human rhinovirus and respiratory syncytial virus in infancy. J Infect Dis 2016; 214: 1924–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Metersky ML, Masterton RG, Lode H, et al. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis 2012; 16: e321–e331. [DOI] [PubMed] [Google Scholar]
- 53. Hanada S, Pirzadeh M, Carver KY, et al. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 2018; 9: 2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yildiz S, Mazel-Sanchez B, Kandasamy M, et al. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 2018; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. He Y, Wang J, Li F, et al. Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections. Front Microbiol 2020; 11: 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fan J, Li X, Gao Y, et al. The lung tissue microbiota features of 20 deceased patients with COVID-19. J Infect 2020; 81: e64–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baghbani T, Nikzad H, Azadbakht J, et al. Dual and mutual interaction between microbiota and viral infections: a possible treat for COVID-19. Microb Cell Fact 2020; 19: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bassetti M, Kollef MH, Timsit J-F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med 2020; 46: 2071–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Srinath BS, Shastry RP, Kumar SB. Role of gut-lung microbiome crosstalk in COVID-19. Res Biomed Eng. Epub ahead of print 24 November 2020. DOI: 10.1007/s42600-020-00113-4. [DOI] [Google Scholar]
- 60. De Maio F, Posteraro B, Ponziani FR, et al. Nasopharyngeal microbiota profiling of SARS-CoV-2 infected patients. Biol Proced Online 2020; 22: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang R-D, Liu M-Q, Chen Y, et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 2020; 182: 50–58.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Israelow B, Song E, Mao T, et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. bioRxiv, 2020. DOI: 10.1101/2020.05.27.118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dong M, Zhang J, Ma X, et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother 2020; 131: 110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seethy AA, Singh S, Mukherjee I, et al. Potential SARS-CoV-2 interactions with proteins involved in trophoblast functions – an in-silico study. Placenta 2021; 103: 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cevik M, Bamford CGG, Ho A. COVID-19 pandemic—a focused review for clinicians. Clin Microbiol Infect 2020; 26: 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cevik M, Kuppalli K, Kindrachuk J, et al. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020; 371: m3862. [DOI] [PubMed] [Google Scholar]
- 68. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181: 1016–1035.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Suárez-Fariñas M, Tokuyama M, Wei G, et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2–related disease. Gastroenterology 2020; 160: 287–301.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fenizia C, Biasin M, Cetin I, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun 2020; 11: 5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li M, Chen L, Zhang J, et al. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 2020; 15: e0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hein S, Yamamoto SY, Okazaki K, et al. Lysyl oxidases: expression in the fetal membranes and placenta. Placenta 2001; 22: 49–57. [DOI] [PubMed] [Google Scholar]
- 73. Ruiz LA, Báez-Vega PM, Ruiz A, et al. Dysregulation of lysyl oxidase expression in lesions and endometrium of women with endometriosis. Reprod Sci 2015; 22: 1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu X-H, Jia Y, Zhou X, et al. Downregulation of lysyl oxidase and lysyl oxidase-like protein 2 suppressed the migration and invasion of trophoblasts by activating the TGF-β/collagen pathway in preeclampsia. Exp Mol Med 2019; 51: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu Y, Chen P. Coronavirus disease 2019 (COVID-19) in neonates and children from China: a review. Front Pediatr 2020; 8: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. Epub ahead of print 9 November 2020. DOI: 10.1016/S2352-4642(20)30342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen W, Yuan P, Yang M, et al. SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal–fetal interface. Engineering (Beijing) 2020; 6: 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020; 323: 1848–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020; 11: 3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lorenz N, Treptow A, Schmidt S, et al. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J 2020; 39: e212. [DOI] [PubMed] [Google Scholar]
- 81. Peng S, Zhu H, Yang L, et al. A study of breastfeeding practices, SARS-CoV-2 and its antibodies in the breast milk of mothers confirmed with COVID-19. Lancet Reg Health West Pac 2020; 4: 100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gao X, Wang S, Zeng W, et al. Clinical and immunologic features among COVID-19–affected mother–infant pairs: antibodies to SARS-CoV-2 detected in breast milk. New Microbes New Infect 2020; 37: 100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pereira A, Cruz-Melguizo S, Adrien M, et al. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand 2020; 99: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nuriel-Ohayon M, Neuman H, Koren O. Microbial changes during pregnancy, birth, and infancy. Front Microbiol 2016; 7: 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stinson LF, Boyce MC, Payne MS, et al. The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front Microbiol 2019; 10: 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Al Alam D, Danopoulos S, Grubbs B, et al. Human fetal lungs harbor a microbiome signature. Am J Respir Crit Care Med 2020; 201: 1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Parnell LA, Briggs CM, Cao B, et al. Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci Rep 2017; 7: 11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lundgren SN, Madan JC, Emond JA, et al. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018; 6: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Raspini B, Porri D, De Giuseppe R, et al. Prenatal and postnatal determinants in shaping offspring’s microbiome in the first 1000 days: study protocol and preliminary results at one month of life. Ital J Pediatr 2020; 46: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. García-Mantrana I, Selma-Royo M, González S, et al. Distinct maternal microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020; 11: 962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Valdes AM, Walter J, Segal E, et al. Role of the gut microbiota in nutrition and health. BMJ 2018; 361: k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lewis FMT, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol 2017; 129: 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mesa MD, Loureiro B, Iglesia I, et al. The evolving microbiome from pregnancy to early infancy: a comprehensive review. Nutrients 2020; 12: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Amir M, Brown JA, Rager SL, et al. Maternal microbiome and infections in pregnancy. Microorganisms 2020; 8: 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics 2018; 141: e20172437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hyman RW, Fukushima M, Jiang H, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci 2014; 21: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sharon G, Cruz NJ, Kang D-W, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019; 177: 1600–1618.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Woodworth KR. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy — SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020; 395: 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Prince AL, Ma J, Kannan PS, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol 2016; 214: 627.e1–627.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pelzer E, Gomez-Arango LF, Barrett HL, et al. Review: maternal health and the placental microbiome. Placenta 2017; 54: 30–37. [DOI] [PubMed] [Google Scholar]
- 102. Aagaard K, Ma J, Antony KM, et al. The placenta harbors a unique microbiome. Sci Transl Med 2014; 6: 237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest 2020; 130: 4947–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pulinx B, Kieffer D, Michiels I, et al. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur J Clin Microbiol Infect Dis 2020; 39: 2441–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mulvey JJ, Magro CM, Ma LX, et al. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann Diagn Pathol 2020; 46: 151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Pathol 2020; 154: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. Epub ahead of print 22 April 2021. DOI: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal–fetal transmission: a systematic review and meta-analysis. Eur J Med Res 2020; 25: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020; 99: 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baud D, Greub G, Favre G, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020; 323: 2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Richtmann R, Torloni MR, Oyamada Otani AR, et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health 2020; 27: e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yoon SH, Kang J-M, Ahn JG. Clinical outcomes of 201 neonates born to mothers with COVID-19: a systematic review. Eur Rev Med Pharmacol Sci 2020; 24: 7804–7815. [DOI] [PubMed] [Google Scholar]
- 114. Oncel MY, Akın IM, Kanburoglu MK, et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society. Eur J Pediatr. Epub ahead of print 10 August 2020. DOI: 10.1007/s00431-020-03767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 2020; 9: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen Y, Peng H, Wang L, et al. Infants born to mothers with a new coronavirus (COVID-19). Front Pediatr 2020; 8: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol 2020; 223: 911.e1–911.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]