Abstract
Background
Colorectal cancer defined as cancer of the colon or rectum, is the third most frequently diagnosed cancer in men and the second in women, and, according to the World Health Organization database GLOBOCAN, it accounts for nearly 1.4 million new cases annually worldwide. The occurrence of colorectal cancer is associated with nonmodifiable risk factors, including age and hereditary factors, as well as with modifiable environmental and lifestyle factors.
Methods
The study included 800 patients, 400 diagnosed with colorectal cancer and 400 within the control group, who gave their written informed consent to participate in the study. Patients with cancer other than colorectal cancer were randomly selected for control group I, and patients with no cancer diagnosis were selected for control group II. The method used was a case-control study – an observational and analytical study with a control group, conducted among patients of the Clinical Oncology Centre and the Provincial Hospital in the years 2019–2020. The study comparing the exposure was carried out in a group of people who developed the endpoint, that is colorectal cancer, with the exposure in a well-matched group of controls who did not reach the endpoint. Assessment of activity and BMI was used according to WHO recommendations, as well as the expert system. The data were tested for the distribution and the homogeneity of variance was validated before applying the parameter tests. Comparison of quantitative variables between groups was performed using ANOVA.
Results
The mean age of the patients was 64.53 ± 8.86 years, of the control group I – 59.64 ± 9.33 and the control group II – 57.5 (7.83). There was a strong positive association between the incidence of ulcerative colitis and the risk of colorectal cancer (P < .01). Among obese subjects, the risk of developing colorectal cancer was 1.27 (95% CI, 1.06–1.53) compared with nonobese subjects. A strong positive relationship was found between low physical activity converted to metabolic equivalent of MET effort per week and the risk of colorectal cancer (P < .001). The relative risk for current smokers was 2.17 (95% CI 1.79–2.66). There was an association between higher fat consumption and higher red meat consumption and the risk of developing colorectal cancer (P < .01).
Conclusions
Obesity, low physical activity, active and passive smoking and high salt and red meat consumption have been associated with an increased risk of colorectal cancer. These findings provide further evidence of the importance of maintaining a healthy lifestyle.
Keywords: colorectal cancer, risk factors, cancer risk, environmental factors, genetic factors
Introduction
Colorectal cancer (CRC), defined as cancer of the colon or rectum, for over 40 years, has become an increasing problem in developed countries, where it is one of the leading neoplastic locations. Recent reports show that the burden is also increasing in most low- and middle-income countries, possibly as a result of urbanisation and the increased incidence of risk factors associated with Western lifestyles.1,2 In 2017, there were 1.8 million (95% UI 1.8–1.9) cases of colorectal cancer worldwide, with an age-standardised incidence rate of 23.2 (22.7–23.7) per 100 000 person-years, which increased by 9.5% (4.5-13.5) in the years 1990–2017. Colorectal cancer in 2017 caused 896 000 (876 300–915 700) deaths worldwide. Slovakia, the Netherlands and New Zealand had the highest standardised incidence rates in 2017, while Greenland, Hungary and Slovakia had the highest standardised death rates. The number of cases and deaths was higher in men than in women aged up to 80–84, with the highest rates observed in the oldest age group (≥95 years) for both sexes in 2017. 3 In 2018, there were 1 849 518 (10.2% of all cancers) colorectal cancer cases worldwide, causing 880 792 (9.2%) deaths. 4 There are estimated 1.93 million new CRC cases diagnosed, and .94 million CRC caused deaths in 2020 worldwide, representing 10% of the global cancer incidence (total 19.29 million new cases) and 9.4% of all cancer caused deaths (total 9.96 million deaths). 5 Incidence rates are highest in Western Europe and North America and lowest in developing countries in Africa and Asia. It is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimate of the number of colorectal cancer cases in the United States in 2020 indicates 104 610 new cases of colon cancer and 43 340 new cases of rectal cancer.1,2 In the 1960s, Poland was a country with very low mortality from these cancers – the mortality rates were in the range of 5–6/105. Changes in the frequency of colorectal cancer within 30 years made Poland a country with a high risk of dying from colorectal cancer (mortality rate 20/105 in men, 10/105 in women). The time trend of the mortality rates for malignant colorectal neoplasms in Poland, especially in men, is characterised by one of the highest upward trends among European countries. Every year, more than 13 000 Poles develop colorectal cancer, of which over 9000 dies. The lifetime risk of developing colorectal cancer is approximately 1 in 23 (4.4%) for men and 1 in 25 (4.1%) for women. 6 The global burden of CRC is expected to increase by 60% to more than 2.2 million new cases and 1.1 million deaths annually by 2030. This increase will be the result of economic, social, environmental and generational changes in developed countries.7-9
The aetiology of colorectal neoplasms has not been fully explained and the immediate causes are still unknown, but many years of research have allowed us to distinguish many risk factors. The occurrence of colorectal cancer is associated with nonmodifiable risk factors, including age and hereditary factors, as well as modifiable factors related to the environment and lifestyle.10–12 The age of the patient is considered to be the main cause. Although cancer occurs also in young people the chance of developing cancer increases after the age of 50 and 9 out of 10 people who develop cancer are over 50 years of age. The peak incidence occurs after the age of 70. Past inflammatory diseases are another risk factor for colorectal cancer. The risk of developing the disease increases 20 times in ulcerative colitis and 3 times in Crohn’s disease. 3 Many risk factors are related to lifestyle. Scientific research indicates low physical activity, a low-residue diet rich in fat, high in calories, rich in red meat, but also low in calcium or folic acid. Besides, alcohol consumption and smoking are also mentioned, which in the US are associated with 1/5 of intestinal cancers. Strong scientific evidence shows that obese people have a greater risk of developing colorectal cancer, and that risk increases with increasing BMI. Elderly people with BMI>30 have a 5–100% higher risk of developing the disease compared to people with BMI<23.13–15 It is also important to pay attention to the genetic background, especially familial adenomatous polyposis (FAP), which is associated with a 100% lifetime risk of developing colorectal cancer, and hereditary nonpolyposis colorectal cancer (HNPCC), where the risk is 70–80%.3,16,17
Today, many scientific reports present risk factors for colorectal cancer, but less research has been done on the strength of individual factors and the interactions between specific risk factors and cancer risk. In this study, we compared 3 groups of patients to investigate the relationship of demographic, environmental and lifestyle factors to the risk of developing colorectal cancer. In the literature, we did not find similar studies that used 2 control groups. We wanted to compare the results obtained in the group of patients with colorectal cancer in relation to patients with cancer other than colorectal cancer and patients without a cancer diagnosis. Our research is innovative in this area, and the obtained results will allow us to state whether it would have any scientific significance in the future. Data on differences in levels and trends in colorectal cancer are needed to understand the impact of each risk factor.
Objective of the Work
The aim of the study is to identify and evaluate patient characteristics, demographic and lifestyle factors that are associated with colorectal cancer at diagnosis.
Data and Method
Study Design
The case-control study, which was an observational and analytical study with a control group, was conducted among patients of the Podkarpackie Clinical Oncology Center and the Provincial Hospital in Rzeszów in 2019–2020. The study comparing the exposure was carried out in a group of people who developed the endpoint, that is colorectal cancer, with the exposure in a well-matched group of controls who did not reach the endpoint. The reference population from which study participants were recruited into both the case and control groups met the following common criteria: they resided in the same geographic area, were treated in the same healthcare institution and were in the 34–85 age group. The study group included patients diagnosed with histopathologically confirmed colorectal cancer, patients in the control group diagnosed with cancer other than colorectal cancer and patients with a different disease entity that was not cancer. The selection of 2 control groups for the group of cases made it possible to simultaneously analyse many potential causal factors and use methods to eliminate their mutual influences. Due to the small sample size, the proportion of patients with fairly consistent characteristics was important. The size of the sample resulted from the limited time of the study, which is preliminary and at this stage is to be used to develop an expert system, facilitating the identification of people from risk groups, during a routine interview conducted at every level of the health care system. The developed system will be tested in subsequent studies on a wider group of patients.
Eligible patients received an information pack from a member of the research group. The information package consisted of a letter describing the objectives of the study and its course, a consent form to be completed if patients were interested in the study and a refusal sheet. After giving their written informed consent, the patient chose to participate in a face-to-face interview conducted in the clinic by an interviewer who was a member of the research group or to fill in the interview form online. The interview lasted approximately 40 minutes. The direct interview, in the case of the patient’s fatigue, was divided into parts to maintain the physical and mental comfort of the patient. The online interview could be completed at any time by the patient.
Participant Recruitment, Inclusion and Exclusion Criteria
The inclusion criterion common to the group of cases and controls was the place of residence in the Podkarpackie Province, treatment at the Provincial Clinical Hospital in Rzeszów or the Podkarpackie Oncology Center, and the age of 34–85 years. Eligible participants were randomly selected from the general population.
All types of histopathologically confirmed colorectal cancer cases, regardless of their stage, were included in the study. The main indicators of participation in the study were the diagnosis of cancer at least 3 months before the study, life expectancy >6 months, age over 18 and awareness of the diagnosis. Patients who did not express their willingness to participate in the study, the ones covered by palliative care and those diagnosed within the last 3 months were excluded from the study because the initial period of diagnosis and treatment is associated with a huge psychological burden and the need to adapt to the new situation and that could introduce errors in the results. Patients who were too physically ill, too emotionally stressed, under the age of 18 or unable to read in Polish were also excluded.
Patients with cancer other than colorectal cancer were randomly selected for control group I, and patients with no cancer diagnosis were selected for control group II.
Sample
The study included 800 patients, 400 diagnosed with colorectal cancer, 200 from the control group I and 200 from the control group II. The mean age of the patients was 64.53 ± 8.86 years, the control group I 59.64 ± 9.33 and the control group II 57.5 (7.83).
Questionnaire for the Patient
The method used in the research was a clinical, direct, individual, structured interview, which was in-depth and focused. The qualitative interview questionnaire was a standardised measuring instrument and was verified by testing a group of 30 patients during the month. The questionnaire contained open-ended, single and multiple-choice questions to obtain demographic, epidemiological, lifestyle and risk behaviour information, as well as risk factors.
Body Mass Index Assessment
Body mass index (BMI) expressed in kg/m2 was calculated from the baseline height and weight and divided into 3 categories according to the WHO standard: normal (<25 kg/m2), overweight (25–29.9 kg/m2) and obesity (>/30 kg/m2).
Assessment of Physical Activity
Assessment of activity according to WHO recommendations was used: undertaking at least moderate activity for about 30 minutes 5 times a week, moderate or intense physical effort performed for at least 45 minutes on at least 5 days a week, undertaking 18–27 hours of metabolic effort (MET) equivalent per week (hour of jogging, cycling, tennis, swimming – 7 MET, an hour of aerobics, lawn mowing – 6 MET, walking for an hour 6 days a week – 18 MET).
Assessment of Covariates
Potential confounders of colorectal cancer risk were selected based on published evidence from European Prospective Investigation into Cancer and Nutrition (EPIC), the International Agency for Research on Cancer (IARC) and the World Cancer Research Fund (WCRF), which included: smoking (never smoking, passive smoking, active smoking, age at which they started and stopped smoking, and number of cigarettes smoked), weekly alcohol consumption based on a relatively safe portion of pure alcohol per day for women (10 g) and for men (20 g), that is one glass of wine and a glass of beer or a small glass of strong alcohol. A portion is 30 mL of vodka (40%/vol.), 100 mL of wine (12%/vol.), 285 mL of strong beer (4.9%/vol.) or 375 mL of light beer (3.5%/vol.), compliance 0 g per day, consumption of 600–800 g with fruit and vegetable guidelines defined as eating more than 2 portions of fruit and 5 portions of vegetables per day or 400–800 g of fibre per day, consumption of red meat less than 80 g per day or 500 g per week.
Expert System
The research method was an individual analysis of cancer risk performed with the use of a computer application based on an expert system. The tools included a skeleton expert system Jess, allowing the formation of decision rules and conclusions. The graphic interface was created with the use of a programming language JavaFx, which allows creation of advanced forms using the CSS styles.
Ethical Considerations
The study was approved by the Ethics Committee at the University of Rzeszów (Resolution No.1 December 2019). Participation in the study was voluntary and anonymous, and the respondents were informed of their right to refuse or withdraw from the study at any time. Each participant was informed about the study objective and the time of study termination.
Data Analysis
Data analysis was performed with the SPSS statistical package version 15.0 for Windows.
Descriptive analysis, bivariate and multivariate logistic regression models were carried out. The adjusted odds ratio (AOR) was used to determine the association between the dependent variable and independent variables with a statistically significant level at a 95% confidence interval (CI). The data were tested for the distribution and the homogeneity of variance was validated before applying the parameter tests. Comparison of quantitative variables between groups was performed using ANOVA/Kruskal-Wallis Test. Quantitative comparison of the variables between the groups was done using the unpaired t/Mann-Whitney test. The comparison of qualitative variables between the groups was performed using the exact Chi-square/Fisher test. Statistical significance was used at the conventional 5% level (P < .05).
Results
Demographic Data
A total of 800 patients participated in the study with a 100% response rate. The study group consisted of 400 cases, 200 of the control group I and 200 of the control group II. The mean age of the patients was 64.53 ± 8.86 years, while in the control group I 59.64 ± 9.33 and in group II 57.5 (7.83). Increasing age was strongly associated with the risk of developing colorectal cancer. The incidence of colorectal cancer correlated with both the place of residence (P < .01) and the level of education (P < .01). Multivariate logistic regression showed that the overall incidence of colorectal cancer was significantly higher among those with low educational attainment (AOR = 1.86; 95% CI: 1.26, 2.75) and those in the countryside (AOR = 0, 45; 95% CI: 0.30, 0.67) compared to the corresponding groups. Table 1 presents other descriptive statistics identifying the studied group.
Table 1.
Descriptive Statistics of the Examined Group of Patients.
| Demographic information | ||||
|---|---|---|---|---|
| Characteristics % (N) | Cases (n 400) | Controls I (n = 200) | Controls II (n = 200) | P |
| Sex | ||||
| Women | 40% (160) | 37% (74) | 88% (176) | .21 |
| Men | 60% (240) | 63% (126) | 12% (24) | |
| The age of the study group | ||||
| SD | 64.53 (8.86) | 59.64±9.33 | 57.5 (7.83) | .12 |
| 95%CI | <34; 82> | <34; 79> | <35; 85> | |
| Place of residence | ||||
| City | 27% (108) | 49% (98) | 36% (72) | .01 |
| Village | 73% (292) | 51% (102) | 64% (128) | |
| Financial situation | ||||
| Very good | 7% (28) | 4% (8) | 20% (40) | .19 |
| Good | 20% (80) | 30% (60) | 51% (102) | |
| Average | 61% (244) | 60% (120) | 20% (40) | |
| Bad | 12% (48) | 6% (12) | 9% (18) | |
| Age groups | ||||
| 34–44 | 1% (4) | 3% (6) | 15% (30) | .07 |
| 45–55 | 6% (24) | 11% (22) | 12% (24) | |
| 56–66 | 53% (212) | 37% (74) | 27% (54) | |
| 67–77 | 33% (132) | 12% (24) | 23% (46) | |
| 78–88 | 7% (28) | 37% (74) | 23% (46) | |
| Education of the study group | ||||
| Higher education | 10% (40) | 23% (46) | 20% (40) | .01 |
| Secondary education | 20% (80) | 46% (92) | 71% (142) | |
| Vocational education | 43% (172) | 23% (46) | 5% (10) | |
| Primary education | 27% (108) | 8% (16) | 4% (8) | |
| Marital status | ||||
| Married | 19% (76) | 54% (108) | 65% (130) | .62 |
| Widowed | 70% (280) | 12% (24) | 11% (22) | |
| Unmarried | 11% (44) | 34% (68) | 24% (48) | |
| Source of income | ||||
| Professionally active | 33% (132) | 31% (62) | 65% (130) | .59 |
| Annuity | 13% (52) | 20% (40) | 12% (24) | |
| Unemployed | 7% (28) | 18% (36) | 9% (18) | |
| Retirement | 47% (188) | 31% (62) | 14% (28) | |
Family History of Neoplastic Diseases
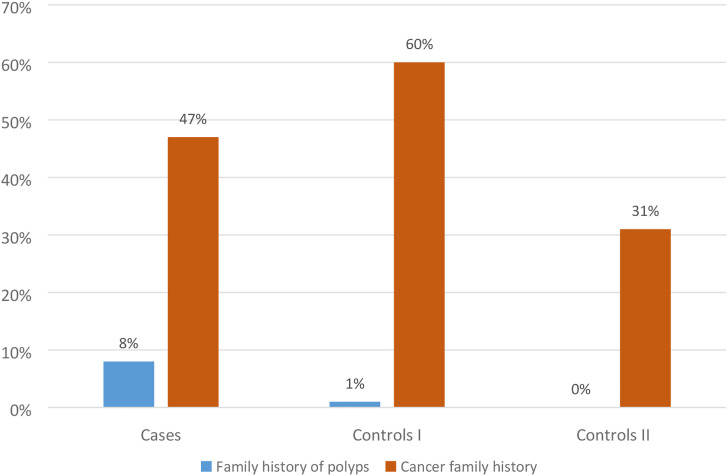
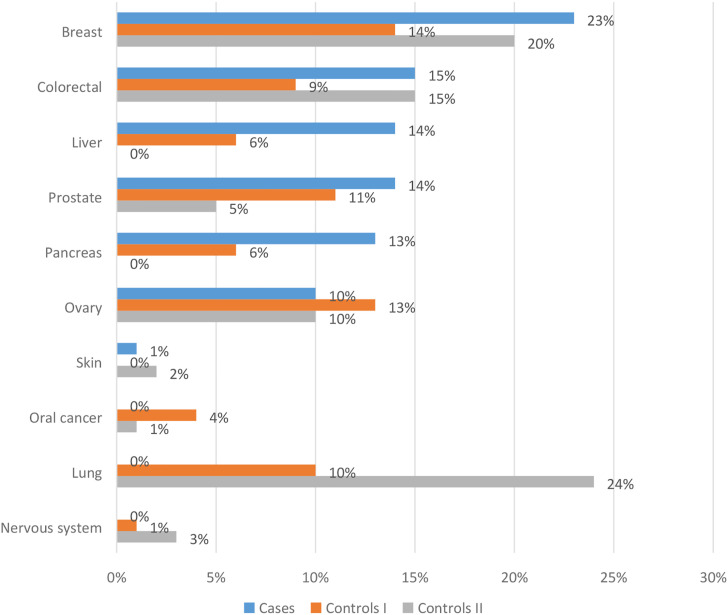
When assessing the incidence of neoplastic diseases in the families of the respondents, it was shown that in the group of patients 47% reported the presence of neoplastic diseases, in the control group I 60% and in group II 31%. Family history of neoplastic diseases was not significantly associated with the risk of colorectal cancer (Figures 1 and 2).
Figure 1.
The occurrence of neoplasms in the respondents’ family.
Figure 2.
The occurrence of neoplasms in the respondents’ family.
Personal Medical History
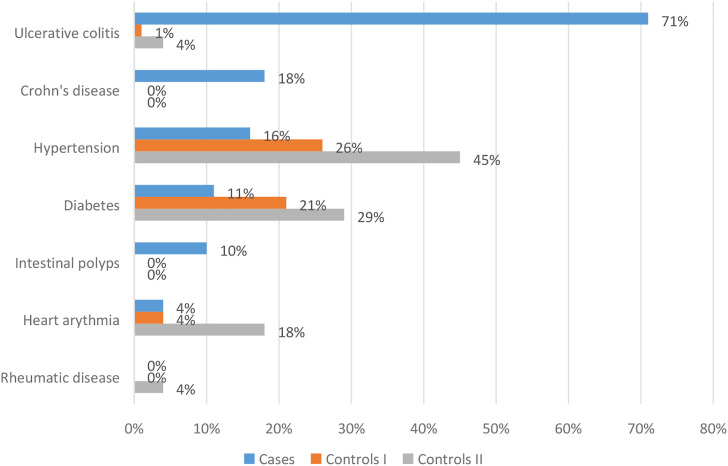
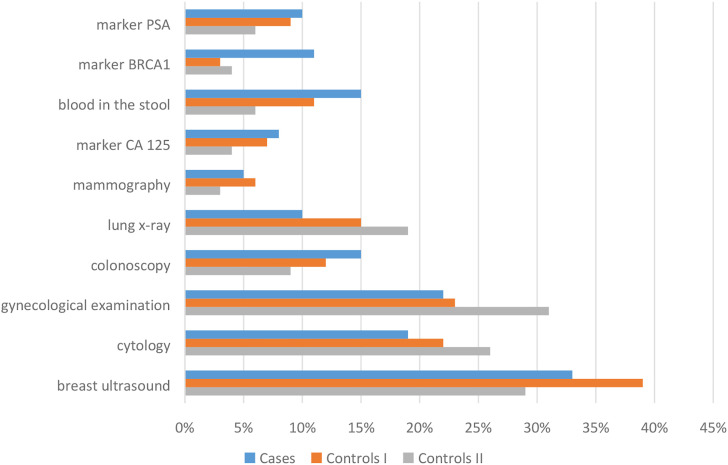
An important element of the analysis was the assessment of the occurrence of diseases increasing the risk of colorectal cancer among the studied patients. There was a strong positive association between the incidence of ulcerative colitis and the risk of colorectal cancer (P < .01) (Figure 3). Patients were asked to perform preventive examinations up to 5 years back. Regular prophylactic examinations were performed on 41% of patients, 57% in the control group I and 46% in the control group II. Among the prophylactic tests performed by patients, there are also cancer markers, defined as high-molecular substances present in the blood, urine or attached to the surface of cancer cells, the identification and measurement of which allow diagnosing patients and planning therapy. The most frequently determined marker in the group of cases was BRCA1 (11%) (Figure 4). During the assessment of the frequency of colonoscopy, it was shown that the largest group of patients underwent examination every 10 years (8% of patients, 9% in the control group I and 6% in the control group II), then every 5 years (5% of patients, 2% in the control group I and 3% in the control group II) and every 2 years (6% of patients, 1% in the control group I and 0% in the control group II). Although patients from the group of cases diagnosed previously with ulcerative colitis had indications to perform colonoscopy every 2 years, according to the procedure in force in Poland, they did not undergo this examination. Therefore, the group of patients with incidence of previous adenomas accounted for 5%.
Figure 3.
History of illness among the respondents.
Figure 4.
Performing screening tests among respondents.
Bodyweight and Physical Activity
60% of patients had BMI greater than 25 kg/m2, 20% in control group I and 35% in group II. Increasing BMI was associated with an increased risk of colorectal cancer (P < .01). Among obese subjects, the risk of developing colorectal cancer was 1.27 (AOR = 1.27; 95% CI, 1.06–1.53) compared with nonobese subjects. Of the patients, 29% did not meet the guidelines for moderate activity, 47% did not meet the guidelines for vigorous activity, with control group II being more likely to follow them. Patients and subjects from the control group I spent more hours a day in a sitting position. A strong positive relationship was found between low physical activity converted to metabolic equivalent of MET effort per week and the risk of colorectal cancer (P < .001) (Table 2).
Table 2.
Lifestyle of the Respondents.
| Variables | ||||
|---|---|---|---|---|
| Characteristics/% (N) | Cases (n = 400) | Controls I (n = 200) | Controls II (n = 200) | P |
| BMI | ||||
| <25 | 40% (160) | 80% (160) | 65% (130) | .71 |
| 25.0–29.9 | 25% (100) | 14% (28) | 23% (46) | .59 |
| ≥30 | 35% (140) | 6% (12) | 12% (24) | .01 |
| Metabolic equivalent of MET effort per week | ||||
| <10 MET | 82% (328) | 62% (124) | 51% (102) | .01 |
| 10–17 MET | 9% (36) | 31% (62) | 29% (58) | .59 |
| 18–27 MET | 9% (36) | 7% (14) | 20% (40) | .14 |
| Weekly activity time | ||||
| Lack of activity | 29% (116) | 24% (48) | 9% (18) | .53 |
| 5 days a week for 30 minutes | 47% (188) | 38% (76) | 67% (134) | .16 |
| 5 days a week for 1 hour | 9% (36) | 31% (62) | 15% (30) | .16 |
| 7 days a week for 30 minutes | 9% (36) | 7% (14) | 9% (18) | .32 |
| Sedentary hours/day | ||||
| 1 –2 | 38% (152) | 32% (64) | 72% (144) | .53 |
| 3–5 | 32% (128) | 41% (82) | 22% (44) | .53 |
| 6–8 | 11% (44) | 7% (14) | 4% (8) | .10 |
| >8 | 19% (76) | 20% (40) | 2% (4) | .53 |
| Stress | ||||
| Acute | 31% (124) | 9% (18) | 27% (54) | .73 |
| Chronic | 20% (80) | 20% (40) | 53% (106) | .88 |
Substance Use
30% of patients were smokers, 21% in control group I and 8% in control group II. Only the group of patients among the respondents chewed tobacco leaves (7%). The lowest age at the start of smoking was 14 years in the group of patients (7%). Patients and control group I were significantly more often smokers (P < .01), and those groups started smoking at an early age (P < .03) significantly more often. The risk of colorectal cancer was significant when smoking more than 30 cigarettes a day (P < .01). Patients who started smoking at an early age (AOR = 2.83; 95% CI: 1.49, 5.37) and smoked more than 30 cigarettes a day (AOR = 2.12; 95% CI: 1.15, 3, 93) had a 2.8 and 2.1 times greater risk of developing cancer. Alcohol consumption was not significantly associated with the risk of colorectal cancer (Table 3).
Table 3.
Lifestyle of the Respondents.
| Variables | ||||
|---|---|---|---|---|
| Characteristics/% (N) | Cases (n = 400) | Controls I (n = 200) | Controls II (n = 200) | P |
| Smoking | ||||
| Never | 16% (64) | 37% (74) | 58% (116) | .94 |
| Passive smoking | 40% (160) | 46% (92) | 7% (14) | .94 |
| An active smoker | 30% (120) | 21% (42) | 8% (16) | .01 |
| Former smoker | 47% (188) | 31% (62) | 27% (54) | .94 |
| Age at the start of regular smoking | ||||
| 10–14 years | 7% (28) | 0% (0) | 0% (0) | .03 |
| 15–20 years | 33% (132) | 20% (40) | 4% (8) | .59 |
| 21–25 years | 7% (28) | 3% (6) | 8% (16) | .52 |
| >25 years | 53% (212) | 77% (154) | 88% (176) | .94 |
| Time of smoking a cigarette | ||||
| <10 years | 7% (28) | 55% (110) | 25% (50) | .62 |
| 10–20 years | 20% (80) | 14% (28) | 64% (128) | .65 |
| >20 years | 73% (292) | 31% (62) | 11% (22) | .01 |
| Number of cigarettes smoked | ||||
| Up to 10 cigarettes a day | 7% (28) | 16% (32) | 12% (24) | .12 |
| From 10 to 20 a day | 20% (80) | 43% (86) | 70% (140) | .59 |
| Over 30 a day | 73% (292) | 41% (82) | 18% (36) | .01 |
| Consuming alcohol | ||||
| Abstinent | 33% (132) | 43% (86) | 39% (78) | .94 |
| 30 mL of vodka daily | 14% (56) | 3% (6) | 4% (8) | .12 |
| 100 mL of wine daily | 14% (56) | 9% (18) | 7% (14) | .52 |
| 380 mL of beer a day | 0% (0) | 9% (18) | 10% (20) | .94 |
| 60 mL of vodka daily | 0% (0) | 9% (18) | 2% (4) | .94 |
| 200 mL of wine a day | 14% (56) | 6% (12) | 24% (48) | .59 |
| 700 mL of beer a day | 14% (56) | 6% (12) | 6% (12) | .12 |
| More | 11% (44) | 15% (30) | 8% (16) | .59 |
Diet
There were no significant associations between the consumption of vegetables and fruits and the way food was prepared. However, the daily consumption of fat in the diet was declared by as many as 87% of the patients, and in control group I by 54% of the respondents and in group II by 51%. A high salt intake was also observed in the group of patients (89%) and control group I (93%). There was an association between higher fat consumption and higher red meat consumption and the risk of developing colorectal cancer (P < .01) (Table 4).
Table 4.
Eating Habits among the Respondents.
| Variables | ||||
|---|---|---|---|---|
| Characteristics/% (N) | Cases (n = 400) | Controls I (n = 00) | Controls II (n = 200) | P |
| Fresh vegetables, fruits | ||||
| Several times every day | 47% (188) | 23% (46) | 41% (82) | .18 |
| Daily once a day | 20% (80) | 37% (74) | 29% (58) | .21 |
| Often several times a week | 33% (132) | 40% (80) | 30% (60) | .17 |
| Preparation of dishes | ||||
| Boiled/steamed | 47% (188) | 37% (74) | 57% (114) | .20 |
| Fried | 20% (80) | 30% (60) | 18% (36) | .55 |
| Baked | 13% (52) | 30% (60) | 26% (52) | .73 |
| Grilled | 20% (80) | 3% (6) | 5% (10) | .71 |
| Fatty meals | ||||
| Daily | 87% (348) | 54% (108) | 51% (102) | .01 |
| A few times a week | 7% (28) | 31% (62) | 33% (66) | .41 |
| Several times a month | 6% (24) | 15% (30) | 16% (32) | .37 |
| Consumption of salt | ||||
| <6 g/day | 11% (44) | 7% (14) | 27% (54) | .91 |
| >6 g/day | 89% (356) | 93% (186) | 73% (146) | .01 |
| Consumption of red meat | ||||
| <80 g/ day or 500 g/week | 13% (52) | 46% (92) | 66% (132) | .82 |
| >80 g/ day or > 500 g/week | 87% (348) | 54% (108) | 34% (68) | .01 |
Discussion
The latest reports, and most of all forecasts for the future, were the basis for starting our research. This study looked at the patient’s characteristics, demographic and lifestyle factors that are associated with colorectal cancer at diagnosis. Our study provided an opportunity to evaluate both nonmodifiable and modifiable risk factors, and one of the strengths of our study was the inclusion of 2 control groups, which allowed for better comparisons and visualisation of differences.
Among the nonmodifiable factors that we assessed was age, which is a major risk factor for sporadic CRC. Colorectal cancer is rare before the age of 40, the incidence begins to increase significantly between the ages of 40 and 50, and incidence rates increase with each subsequent decade. More than 90% of colorectal cancer cases occur in people over the age of 50. The incidence is over 50 times higher in people aged 60–79 than in people under 40.12,18 The above data were confirmed by our research that shows that a clear peak incidence occurred in the age groups of 56–66 (53%) and 67–77 (33%), which was emphasised by the results regarding the age of onset in the control groups. Our results show the continuing trend in the United States, where incidence of diagnosis with CRC in a group of people aged over 65 is about 3 times more likely than people aged 50–64 and about 30 times more likely than people aged 25–49,19,20 although, the most recent data from the United States Surveillance, Epidemiology and End Results (SEER) database suggest that the incidence of CRC is increasing in the under 50 age group while it is decreasing in older groups. In the United States, the incidence of CRC in men and women under the age of 50 continued to increase at a rate of 2% annually from 1995 to 2016. Some registries report an increased incidence of CRC even among young adults aged 20–39 years, although the absolute number of cases in this age group remains significantly lower than that of adults aged 50 years and older.21–30 Research by O’Connell and Fairley confirms these results.31-33
Our studies also attempted to assess the effect of sex on the risk of colorectal cancer. In the group of cases, the difference between the sex was visible (40% of women and 60% of men), but the greatest difference was in the control group I (37% of women and 63% of men). Although the difference in incidence in the group of cases was not statistically significant, it confirmed international reports. Across all age groups and nations, males are approximately 1.5 times more likely to develop CRC than females, and mortality is approximately 25% higher in males than females.34,35 Most cases of colorectal cancer occur in people with no family history of colorectal cancer or a predisposing disease, as our research confirms. In our study, none of the patients had a diagnosis of congenital syndrome predisposing to colorectal cancer, and no such burden was found in the assessment of oncological pedigree. Family history of cancer was not significantly associated with the risk of colorectal cancer. The incidence of neoplastic diseases in total concerned 47% of the subjects, colorectal neoplasms 15% and colorectal polyps 8%, and these results were comparable to the results of the control groups. Nevertheless, up to 30% of people who develop bowel cancer have other family members affected by the disease. People with a history of colorectal cancer or adenomatous polyps in one or more first-degree relatives are at increased risk.12,18
We also assessed the impact of other diseases, including gastrointestinal diseases, on cancer risk. Neoplastic colon polyps are precursors of colorectal cancer. The lifetime risk of developing colorectal adenoma in the US population is almost 19%. Almost 95% of sporadic colorectal cancers develop from these adenomas. A subject with a history of adenomas has an increased risk of developing colorectal cancer than those with no previous history of adenomas.12,18 Besides, patients with chronic inflammatory bowel disease have a doubled risk of developing CRC. The relative risk of colorectal cancer in IBD patients has been estimated from 4 to 20 times. Patients with ulcerative colitis are more likely to have CRC (HR (hazard ratio) 33.3, 95% CI (confidence interval): 23.1–49.1) than patients with Crohn’s disease (HR = 5.8, 95% CI: 3.2–10.4).36-45 Our research confirms the above results. There was a strong positive association between the occurrence of ulcerative colitis and the risk of colorectal cancer. Compared to control groups, colorectal polyps (10%), ulcerative colitis (71%) and Crohn’s disease (18%) were significantly more frequent among patients with colorectal cancer. Other conditions associated with both the case group and the control group were not related to cancer, and this also applies to people with diabetes, which is associated with an increased risk of CRC. 46 As evidenced by the results of the metaanalysis conducted by Yuhar H et al, The risk of colon cancer in diabetics was 38% higher than in nondiabetics, and in the case of rectal cancer by 20% higher. 47 There were no patients in the studied groups with diseases that, as numerous reports have shown, are related to CRC. Increased risk, even 10 times higher, occurs in patients with cystic fibrosis moreover,37,48 an increased incidence is observed in acromegaly, especially in people with the uncontrolled disease, as well as cholecystectomy.49,50
Colorectal cancer is widely regarded as an environmental disease involving a wide range of cultural, social and lifestyle factors.12,18 Low socioeconomic status is associated with an increased risk of developing CRC. In a study by Doubeni et al, it was estimated that the risk of CRC increased by approximately 30% in the lowest socioeconomic status quintile compared to the highest. The overall incidence of CRC was significantly higher among those with low educational attainment or living in low socioeconomic districts.51,52 Very similar results were obtained by Kawakatsu et al, who showed that lower socioeconomic status was associated with an increased risk of gastrointestinal cancer. Compared to people with lower education, people with higher education showed a statistically significantly lower risk of developing colorectal cancer 0.52 (0.38–.71). 53 Our research confirmed the above results. Colorectal cancer mainly affected people with vocational (43%) or primary (27%) education and with an unsatisfactory economic situation. The differences were significant compared to the control groups.
Potentially modifiable behaviours such as physical inactivity, unhealthy diet, smoking and obesity are thought to account for a significant part (30–50%) of the socioeconomic imbalance in the risk of developing CRC. Diet strongly influences the risk of colorectal cancer. A diet high in fat, especially animal fats, high-temperature meal preparation, red meat and diets low in fruit and vegetables are major risk factors for colorectal cancer.12,18 In 2015, the International Agency for Research on Cancer of the World Health Organization (IARC) reviewed the evidence linking the consumption of red and processed meat to CRC and classified the consumption of processed meat as carcinogenic to humans and the consumption of red meat as possibly carcinogenic. This position was echoed in the 2020 report. In 2018, the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) similarly concluded that evidence was convincing that consumption of processed meat increased the risk of CRC, while the evidence of the consumption of unprocessed red meat was classified as probable. It has been estimated that for every 50 g of processed meat consumed daily, the risk of developing CRC increases by about 16%, and for every 100 g of red meat consumed daily, it increases by about 12%. For colon cancer, these estimates were 23% and 22%, respectively. 54 Prospective studies have shown a relative risk (RR) of 1.22 among those who eat the most red and processed meats. 55 A metaanalysis of 60 studies found that consumption of red meat and processed meat increased the overall risk of developing CRC. The CRC with red meat consumption was 1.12 (95% CI: 1.03–1.21), and the RR with processed meat consumption was 1.15 (95% CI: 1.07–1.24). 56 While red meat is rich in fats and inflammatory substances such as omega-6, most carcinogens likely come from high-temperature cooking, curing, and smoking. 57 It should be noted that the data from randomised trials do not agree with the hypothesis that eating red, processed meat increases the risk of developing colorectal cancer. The Women’s Health Initiative has not been able to demonstrate that reducing dietary fat, including animal fat, reduces the risk of CRC after more than 8 years of follow-up.58,59 Our research found no significant correlation between fruit and vegetable consumption and the way food was prepared. However, as many as 87% of patients declared daily consumption of fat in the diet. There was an association between higher fat consumption and higher red meat consumption and the risk of developing colorectal cancer (P < .01).
Both obesity and lack of exercise are the most important behavioural factors in the development of colorectal cancer, and a sedentary lifestyle has been proposed as an independent risk factor in colorectal carcinogenesis. Our studies showed that increasing BMI was associated with an increased risk of colorectal cancer (P < .01). Of the patients, 29% did not meet the guidelines for moderate physical activity and spent more hours per day sitting in a class. According to numerous studies, people with the most sedentary lifestyles have up to a 50% higher risk of developing CRC. Obese men have been found to have a 50% greater risk of colon cancer and a 20% greater risk of rectal cancer. 36 A metaanalysis of 13 cohort studies showed that a 5 kg increase in body weight was associated with a 3% increase in the risk of CRC. 60 In a recent evaluation of observational studies on body obesity and cancer risk, the International Agency for Research on Cancer reported a 30% increased likelihood of developing CRC in those with the highest BMI compared to the lowest. 61 A recent metaanalysis of prospective studies showed a 47% increase in the risk of colon cancer and a 15% increase in rectal cancer, comparing the highest BMI category with the lowest.62,63 An analysis by Nunez et al showed that the group with the highest BMI was associated with an increased risk of colon cancer, 64 and a metaanalysis of data from studies showed that weight gain between early adulthood and middle age was associated with a moderate but significant increase in CRC risk, and weight gain between middle and older adulthood was lower but still statistically significant62,65
The study also assessed the effect of substances on the risk of colorectal cancer. We showed that patients and control group I were significantly more often smokers (P < .01), and that those groups started smoking at an early age (P < .03) significantly more often. In 2009, the IARC concluded that smoking does cause colon cancer. It has been found that the relative risk of CRC with regular smoking is 1.18. 36 A recent metaanalysis of 14 prospective cohort studies found that prior (HR = 1.12; 95% CI: 1.04–1.20) and current smoking (HR = 1.29, 95% CI: 1.04–1.60) were associated with a worse prognosis in CRC compared to nonsmokers and smokers. 66 In a metaanalysis of 106 observational studies, it was estimated that the risk of CRC was increased among cigarette smokers compared to those who had never smoked (RR 1.18, 95% CI 1.11–1.25). 67 The risk of dying from CRC was also increased among smokers (RR 1.25, 95% CI 1.14–1.37). In both morbidity and mortality, the relationship was stronger in rectal cancer than in colon cancer. 68 Passive smoking is also carcinogenic, as smoke from the incandescent tip of a cigarette is 4 times more toxic than smoke from the smoker. In a smoky room, a nonsmoker inhales 3 times more carbon monoxide, more than 10 times more nitrosamines, 15 times more benzene and up to 70 times more ammonia than during active smoking. The American Cancer Society report showed that secondhand smoke increases the risk of developing, among others, anal cancer. 1 As with smoking, regular consumption of alcohol may be associated with an increased risk of developing colorectal cancer. 12 People who drink 2–3 alcoholic drinks a day have a 20% higher risk of developing CRC, while for those over 3 drinks, this risk increases to 40%. 37 Several studies have found an association between alcohol consumption and an increased risk of CRC.69,70 A metaanalysis of 27 cohort studies and 34 case-control studies found that compared to those who never drank, there was a significant increase in the risk of CRC for moderate (2 to 3 drinks a day RR 1.21, 95% CI 1.13–1.28) and heavy drinkers (≥4 drinks a day, RR 1.52, 95% CI 1.27–1.81) but not light drinkers (≤1 drink a day, RR 1.00, 95% CI 0, 95–1.05). 69 These results are consistent with other summary analyses.71-73 However, contrary to previous studies, the dose-response analysis showed a significant 7% increase in the risk of CRC even in light drinkers (RR with 10 g/day ethanol consumption 1.07 95% CI 1.04–1.10 74 Alcohol consumption was not significantly associated with the risk of colorectal cancer in our studies.
Recent epidemiological evidence generally suggests that psychosocial factors, including stress, may be considered risk factors for certain types of cancer, including colorectal cancer.75–80 A case-control study by Azizi and Esmaeila in 4 Iranian hospitals found a link between stressful life events and colon cancer. After taking into account the known risk factors, the authors found a 2.49 times higher risk of colorectal cancer associated with the death of loved ones compared to the control group. 81 Kikuchi et al analysed participants’ data from the Japan Collaborative Cohort Study to measure the relationship between perceived stress assessed and the incidence of colorectal cancer. The authors found a significant association between daily perceived stress of moderate or high/severe intensity and the incidence of rectal cancer, for example a 2.16-fold and 1.75-fold increase in risk in men, respectively, but not in colon cancer. 82
Our study explored the effect of exposure variables on colorectal cancer risk among patients diagnosed with cancer and linked the data to administrative data. The main strengths of this analysis are the use of 2 control groups in the study and a large number of all cases (n = 800). However, there are some limitations to consider. Exposure variables were derived from self-report, however, BMI and physical activity were validated. Our research should be interpreted as exploratory.
In the future, we want to compare the risk of individual risk factors among patients with colorectal cancer from the western parts of Poland. We know from studies by other authors that there is a large geographical difference in the global distribution of colorectal cancer and that colorectal cancer is mainly a disease of developed countries with Western culture. We want to check whether such differences are visible in a smaller territorial range and whether it also applies to the criterion of a given country. We will increase the sample size to reinforce the results in this way. In addition, we would like to use the obtained data to create an algorithm that uses artificial intelligence to verify the potential risk of colorectal cancer in a patient during routine visits to the health care system. In our research, we used traditional statistical analysis techniques, but in the future studies it is possible to apply the data-mining technology mine information to discover knowledge based on the premise of unclear assumptions in connection with public databases. 83
Conclusions
1. When comparing the occurrence of risk factors in 3 groups of patients with and without neoplastic disease, it is easier to notice certain relationships. Neoplasms are more common in men, including colorectal cancer, and among people over 50 years of age. Diseases of the gastrointestinal tract predisposing to colorectal cancer are a significant predictor. An inactive and unhealthy lifestyle is the overall burden of cancer.
2. Much of the socioeconomic difference in the risk of developing colorectal cancer can be attributed to the higher incidence of adverse health behaviours in low-status populations and lower education levels.
3. Obesity, low physical activity, active and passive smoking and high salt and red meat consumption have been associated with an increased risk of colorectal cancer. These findings provide further evidence of the importance of maintaining a healthy lifestyle.
4. The move from identifying theoretically avoidable causes of colorectal cancer to implementing prevention strategies depends on the determination of exposures deemed to be causally related to disease development.
5. This analysis confirms the importance of independently following strict guidelines for physical activity, achieving and maintaining a healthy BMI, and adhering to dietary recommendations.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211056692 for Title: Risk Factors for the Diagnosis of Colorectal Cancer by Anna Lewandowska, Grzegorz Rudzki, Tomasz Lewandowski, Aleksandra Stryjkowska-Góra and Sławomir Rudzki in Cancer Control
Acknowledgements
We are thankful to all the participants in this study. The authors also thank the regional authorities and hospital managements for permission, cooperation, contributions and logistic support during data collection.
Author Contributions: Conceptualisation, A.L.; data curation, A.L., G.R. and T.L.; formal analysis, A.L. and T.L.; funding acquisition, A.L., G.R., S.R., A.SG. and T.L.; investigation, A.L.; methodology, A.L., project administration, A.L., A.SG. and T.L.; resources, G.R., T.L.; software, T.L.; supervision, A.L. and S.R.; validation, A.L. and A.SG.; visualisation, T.L.; writing original draft, A.L.; writing review and editing, A.L.; G.R.; and T.L. All authors have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article. This study was supported by our own resources.
Availability of Data and Materials: The data are not publicly available due to privacy and ethical restrictions. The data presented in this study may be available conditionally from the corresponding author.
Ethical Approval: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by The Bioethics Committee at the University of Rzeszow (Resolution No. December 1, 2019).
Informed Consent: Informed consent was obtained from all subjects involved in the study.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Anna Lewandowska https://orcid.org/0000-0001-7743-5035
Grzegorz Rudzki https://orcid.org/0000-0003-0929-9419
References
- 1.American Cancer Society . Atlanta, Georgia: American Cancer Society; 2021. https://www.cancer.org/. Accessed January 22, 2021. [Google Scholar]
- 2.Fukuda I, Hizuka N, Murakami Y, et al. Clinical features and therapeutic outcomes of 65 patients with acromegaly at Tokyo Women’s Medical University. Intern Med. 2001;40(10):987-992. doi: 10.2169/internalmedicine.40.987. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Colorectal Cancer Collaborators . The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):913-933. doi: 10.1016/S2468-1253(19)30345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodarzi E, Beiranvand R, Naemi H, Momenabadi V, Khazaei Z. Worldwide incidence and mortality of colorectal cancer and human development index (HDI): an ecological study. WCRJ. 2019;6:e1433. doi: 10.32113/wcrj_201911_1433. [DOI] [Google Scholar]
- 5.Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Translat Oncol. 2021;14(10):101174. doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Registry . Cork, Ireland: National Cancer Registry; 2020. http://onkologia.org.pl. Accessed November 7, 2020. [Google Scholar]
- 7.International Agency for Research on Cancer . World Cancer Report. Lyon, France: International Agency for Research on Cancer; 2020. https://www.iarc.fr. Accessed October 9, 2020. [Google Scholar]
- 8.US Department of Health and Human Services, US Department of Agriculture . Dietary Guidelines for Americans. In: US Department of Health and Human Services, US Department of Agriculture. 8th ed. Washington, DC: United States of America; 2015-20202015. [Google Scholar]
- 9.World Cancer Research Fund . American Institute for Cancer Research. Analysing Research on Cancer Prevention and Survival. The Cancer Proces. London, UK: World Cancer Researcg Fund; 2018. https://www.wcrf.org. Accessed December 15, 2020. [Google Scholar]
- 10.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi: doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785-2800. doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggar F, Boushey R. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2018. [Google Scholar]
- 15.World Health Organization International Agency for Research on Cancer . Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monographs Evaluat Carcinogen Risks Humans. 2012;100:1-538. [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization International Agency for Research on Cancer . Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monographs Evaluat Carcinogen Risks Humans. 2007;89:1-592. [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Cancer. Geneva, Switzerland: World Health Organization. https://www.who.int/en/news-room/fact-sheets/detail/cancer. Accessed December 15, 2020. [Google Scholar]
- 18.Amersi F, Agustin M, Ko CY. Colorectal cancer: epidemiology, risk factors, and health services. Clin Colon Rectal Surg. 2005;18(3):133-140. doi: 10.1055/s-2005-916274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute . SEER Explorer: An Interactive Website for SEER Cancer Statistics Surveillance Research Program. Bethesda, MD: National Cancer Institute. Available at: https://seer.cancer.gov/explorer/. Accessed November 10, 2020. [Google Scholar]
- 21.Brenner DR, Heer E, Sutherland RL, et al. National trends in colorectal cancer incidence among older and younger adults in Canada. JAMA Network Open. 2019;2(7):e198090. doi: 10.1001/jamanetworkopen.2019.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abualkhair WH, Zhou M, Ahnen D, Yu Q, Wu X-C, Karlitz JJ. Trends in incidence of early-onset colorectal cancer in the United States among those approaching screening age. JAMA Network Open. 2020;3(1):e1920407. doi: 10.1001/jamanetworkopen.2019.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meester RGS, Mannalithara A, Lansdorp-Vogelaar I, Ladabaum U. Trends in incidence and stage at diagnosis of colorectal cancer in adults aged 40 through 49 years, 1975-2015. J Am Med Assoc. 2019;321(19):1933-1934. doi: 10.1001/jama.2019.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward EM, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20-49 years. JNCI: J Nat Can Inst. 2019;111(12):1279-1297. doi: 10.1093/jnci/djz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montminy EM, Zhou M, Maniscalco L, et al. Contributions of adenocarcinoma and carcinoid tumors to early-onset colorectal cancer incidence rates in the United States. Ann Intern Med. 2021;174(2):157-166. doi: 10.7326/M20-0068. [DOI] [PubMed] [Google Scholar]
- 26.Howren A, Sayre EC, Loree JM, et al. Trends in the incidence of young-onset colorectal cancer with a focus on years approaching screening age: A population-based longitudinal study. JNCI: J Nat Can Inst. 2021;113(7):863-868. doi: 10.1093/jnci/djaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA A Cancer J Clin. 2020;70(3):145-164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 28.Singh KE, Taylor TH, Pan C-JG, Stamos MJ, Zell JA. Colorectal cancer incidence among young adults in California. J Adolesc Young Adult Oncol. 2014;3(4):176-184. doi: 10.1089/jayao.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tawadros PS, Paquette IM, Hanly AM, Mellgren AF, Rothenberger DA, Madoff RD. Adenocarcinoma of the rectum in patients under age 40 is increasing. Dis Colon Rectum. 2015;58(5):474-478. doi: 10.1097/DCR.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 30.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. JNCI: J Nat Can Inst. 2017;109(8):djw322. doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69(10):866-872. [PubMed] [Google Scholar]
- 32.Patel SG, Ahnen DJ. Colorectal cancer in the young. Curr Gastroenterol Rep. 2018;20(4):15. doi: 10.1007/s11894-018-0618-9. [DOI] [PubMed] [Google Scholar]
- 33.Fairley TL, Cardinez CJ, Martin J, et al. Colorectal cancer in U.S. adults younger than 50 years of age, 1998-2001. Cancer. 2006-2001;107(5):1153-1161. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 34.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 35.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70(1):96-10824. doi:108.e110.1016/j.gie.2008.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterol Rev. 2019;14(2):89-103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutgens MWMD, van Oijen MGH, van der Heijden GJMG, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(4):789-799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 38.Olén O, Askling J, Sachs M, et al. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964-2014. BMJ. 2017;358:j3951. doi: 10.1136/bmj.j3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins MA, Dowty JG, Ait Ouakrim D, et al. Short-term risk of colorectal cancer in individuals with lynch syndrome: a meta-analysis. J Clin Oncol. 2015;33(4):326-331. doi: 10.1200/JCO.2014.55.8536. [DOI] [PubMed] [Google Scholar]
- 40.Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67(7):1306-1316. doi: 10.1136/gutjnl-2017-314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh M, McBride A, Yun S, et al. BRCA1andBRCA2Gene mutations and colorectal cancer risk: systematic review and meta-analysis. JNCI: J Nat Can Inst. 2018;110(11):1178-1189. doi: 10.1093/jnci/djy148. [DOI] [PubMed] [Google Scholar]
- 42.Katona BW, Stadler ZK, Robson ME, Domchek SM. RE: BRCA1 and BRCA2 Gene mutations and colorectal cancer risk: systematic review and meta-analysis. JNCI: J Nat Can Inst. 2019;111(5):522-523. doi: 10.1093/jnci/djz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cullinane CM, Creavin B, O’Connell EP. Risk of colorectal cancer associated with BRCA1 and/or BRCA2 mutation carriers: systematic review and meta-analysis. Br J Surg. 2020;107(8):951-959. doi: 10.1002/bjs.11603. [DOI] [PubMed] [Google Scholar]
- 44.Ng SC, Lau JYW, Chan FKL, et al. Risk of advanced adenomas in siblings of individuals with advanced adenomas: a cross-sectional study. Gastroenterology. 2016;150(3):608-616. doi: 10.1053/j.gastro.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123-131. doi: 10.1016/S0140-6736(19)32545-0. [DOI] [PubMed] [Google Scholar]
- 46.Ma Y, Yang W, Song M, et al. Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br J Cancer. 2018;119(11):1436-1442. doi: 10.1038/s41416-018-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106(11):1911-1921. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada A, Komaki Y, Komaki F, Micic D, Zullow S, Sakuraba A. Risk of gastrointestinal cancers in patients with cystic fibrosis: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):758-767. doi: 10.1016/S1470-2045(18)30188-8. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Liu H, Li L, et al. Cholecystectomy can increase the risk of colorectal cancer: a meta-analysis of 10 cohort studies. PLoS One. 2017;12(8):e0181852. doi: 10.1371/journal.pone.0181852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagergren J, Ye W, Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology. 2001;121(3):542-547. doi: 10.1053/gast.2001.27083. [DOI] [PubMed] [Google Scholar]
- 51.Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer. Cancer. 2012;118(14):3636-3644. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Nat Can Inst. 2012;104(18):1353-1362. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawakatsu Y, Koyanagi YN, Oze I, et al. Association between socioeconomic status and digestive tract cancers: a case-control study. Cancers. 2020;12(11):3258. doi: 10.3390/cancers12113258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouvard V, Loomis D, Guyton KZ, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599-1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 55.Chan DSM, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Z, Feng Q, Yin Z, et al. Red and processed meat consumption and colorectal cancer risk: a systematic review and meta-analysis. Oncotarget. 2017;8(47):83306-83314. doi: 10.18632/oncotarget.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim E, Coelho D, Blachier F. Review of the association between meat consumption and risk of colorectal cancer. Nutr Res. 2013;33(12):983-994. doi: 10.1016/j.nutres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Lakkis NA, El-Kibbi O, Osman MH. Colorectal cancer in Lebanon: incidence, temporal trends, and comparison to regional and Western Countries. Cancer Control. 2021;28:107327482199686. doi: 10.1177/1073274821996869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the women’s health initiative randomized controlled dietary modification trial. J Am Med Assoc. 2006;295(6):643-654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 60.Karahalios A, Simpson JA, Baglietto L, et al. Change in weight and waist circumference and risk of colorectal cancer: results from the Melbourne collaborative cohort study. BMC Cancer. 2016;16:157. doi: 10.1186/s12885-016-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cong YJ, Gan Y, Sun HL, et al. Association of sedentary behaviour with colon and rectal cancer: a meta-analysis of observational studies. Br J Cancer. 2014;110(3):817-826. doi: 10.1038/bjc.2013.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer - viewpoint of the IARC working Group. N Engl J Med. 2016;375(8):794-798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nunez C, Nair-Shalliker V, Egger S, Sitas F, Bauman A. Physical activity, obesity and sedentary behaviour and the risks of colon and rectal cancers in the 45 and up study. BMC Publ Health. 2018;18:325. doi: 10.1186/s12889-018-5225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol. 2015;181(11):832-845. doi: 10.1093/aje/kwu357. [DOI] [PubMed] [Google Scholar]
- 66.Ordóñez-Mena JM, Walter V, Schöttker B, et al. Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: a meta-analysis of individual patient data from cohorts within the CHANCES consortium. Ann Oncol. 2018;29(2):472-483. doi: 10.1093/annonc/mdx761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer. J Am Med Assoc. 2008;300(23):2765-2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 68.Botteri E, Iodice S, Raimondi S, Maisonneuve P, Lowenfels AB. Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology. 2008;134(2):388-395. doi: 10.1053/j.gastro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958-1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 70.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603-613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 71.Mizoue T, Inoue M, Wakai K, et al. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol. 2008;167(12):1397-1406. doi: 10.1093/aje/kwn073. [DOI] [PubMed] [Google Scholar]
- 72.McNabb S, Harrison TA, Albanes D, et al. Meta‐analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer. 2020;146(3):861-873. doi: 10.1002/ijc.32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine-low-folate diets, and risk of colon cancer in men. J Nat Can Inst. 1995;87(4):265-273. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen LH, Liu P-H, Zheng X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr. 2018;2(4):pky073. doi: 10.1093/jncics/pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kruk J, Aboul-Enein BH, Bernstein J, Gronostaj M. Psychological stress and cellular aging in cancer: a meta-analysis. Oxidat Med Cell Longev. 2019;2019:1-23. doi: 10.1155/2019/1270397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abate M, Citro M, Caputo M, Pisanti S, Martinelli R. Psychological stress and cancer: new evidence of an increasingly strong link. Transl Med. 2020;23:53-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Afrisham R, Paknejad M, Soliemanifar O, Sadegh-Nejadi S, Meshkani R, Ashtary-Larky D. The influence of psychological stress on the initiation and progression of diabetes and cancer. Int J Endocrinol Metabol. 2019;In Press(2):e67400. doi: 10.5812/ijem.67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jia Y, Li F, Liu YF, Zhao JP, Leng MM, Chen L. Depression and cancer risk: a systematic review and meta-analysis. Publ Health. 2017;149:138-148. doi: 10.1016/j.puhe.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 79.Yang T, Qiao Y, Xiang S, Li W, Gan Y, Chen Y. Work stress and the risk of cancer: a meta‐analysis of observational studies. Int J Cancer. 2019;144(10):2390-2400. doi: 10.1002/ijc.31955. [DOI] [PubMed] [Google Scholar]
- 80.Baritaki S, de Bree E, Chatzaki E, Pothoulakis C. Chronic stress, inflammation, and colon cancer: a CRH system-driven molecular crosstalk. J Clin Med. 2019;8(10):1669. doi: 10.3390/jcm8101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azizi H, Esmaeili ED. Stressful life events and risk of colorectal cancer: a case-control study of Iran, 16; 2015:2403-2407. doi: 10.7314/apjcp.2015.16.6.2403.Asian Pac J Cancer Prev APJCP 6 [DOI] [PubMed] [Google Scholar]
- 82.Kikuchi N, Nishiyama T, Sawada T, et al. Perceived stress and colorectal cancer incidence: the Japan collaborative cohort study. Sci Rep. 2017;7:40363. doi: 10.1038/srep40363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu W-T, Li Y-J, Feng A-Z, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Milit Med Res. 2021;8(1):44. doi: 10.1186/s40779-021-00338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211056692 for Title: Risk Factors for the Diagnosis of Colorectal Cancer by Anna Lewandowska, Grzegorz Rudzki, Tomasz Lewandowski, Aleksandra Stryjkowska-Góra and Sławomir Rudzki in Cancer Control