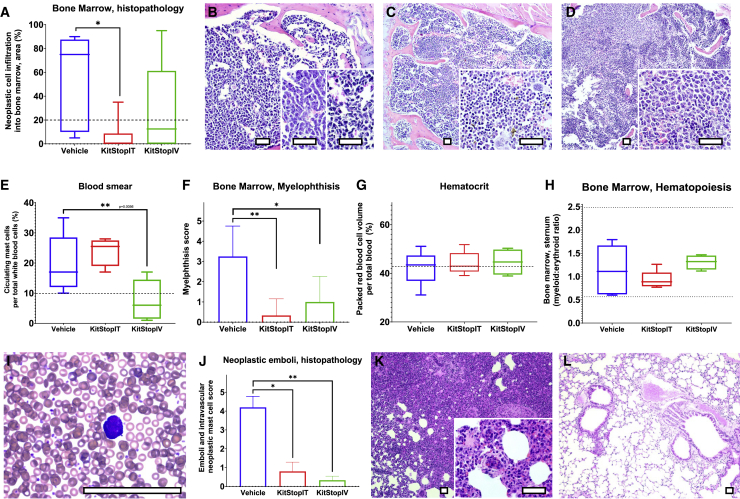
Figure 8.
KitStop ESO administration reduced cardinal signs of mast cell leukemia in an isograft model of mutant mast cell neoplasia
(A) Percent of bone marrow infiltrated by P815 neoplastic cells. (B) Representative micrograph of bone marrow from vehicle controls (B) compared to i.t. (C) and i.v. (D) Kitstop ESO administration. (E) Displacement of hematopoietic bone marrow tissue (myelophthisis) by neoplastic mast cells. (F) Packed red blood cell volume (hematocrit). Some animals from each group had a hematocrit below the lower 2SD reference interval (dotted line) for female DBA/2J mice. (G) Bone marrow hematopoiesis as measured by the myeloid progenitor-to-erythroid progenitor (M:E) ratio. (H) Circulating neoplastic mast cells on differential counts. (I) Blood smear of neoplastic mast cells with Romanowsky stain (representative image shown from vehicle control animal blood). (J) H&E histopathology assessment of neoplastic emboli and circulating neoplastic cells in organs. (K–L) Emboli of neoplastic mast cells in pulmonary vessels from H&E histopathology sections from control (K) and i.v. KitStop ESO (L) treatment groups. (A and F–H) Graphical representation of data includes boxplots where middle bar represents median and whiskers represent minimum and maximum. The p value was determined by a Fisher’s LSD test with an ordinary one-way ANOVA or uncorrected Dunn’s test with a Kruskal-Wallis one-way ANOVA. (E and J) Graphical representation of data includes mean and SEM. The p values were determined independently by a Mann-Whitney test. ∗p ≤ 0.05, ∗∗p ≤ 0.01. Scale bars, 100 μm.