Abstract
Purpose
The programmed death protein 1 (PD-1) pathway is the critical mechanism in development of hepatocellular carcinoma (HCC). The present study analyzed the prognostic impact of pretransplant serum soluble PD-1 (sPD-1) concentration and α-FP–des-γ-carboxyprothrombin–tumor volume (ADV) score in patients with previously untreated HCC undergone liver transplantation (LT).
Methods
This retrospective single-center study enrolled 100 patients with HCC who underwent living donor LT from 2010 to 2016. Concentrations of sPD-1 were measured in stored serum samples.
Results
Receiver operating characteristic curve analysis of 2-year tumor recurrence resulted in an sPD-1 cutoff of 177.1 µg/mL, which was associated with higher rates of tumor recurrence (P = 0.022), but not with overall patient survival (P = 0.460). The derived cutoff for pretransplant ADV score was 5.4log, which was associated with higher tumor recurrence rate (P < 0.001) and lower overall patient survival rate (P < 0.001). Both sPD-1 of >177.1 µg/mL (hazard ratio [HR], 2.26; P = 0.020) and pretransplant ADV score of >5.4log (HR, 3.56; P < 0.001) were independent risk factors for posttransplant HCC recurrence. The combination of these 2 factors enabled the stratification of patients into 3 groups, with groups having 0, 1, and 2 risk factors differing significantly in the prognosis of tumor recurrence (P < 0.001) and overall patient survival (P = 0.006).
Conclusion
Both sPD-1 concentration and ADV score have prognostic impacts in patients who underwent LT for untreated HCCs. These factors, both individually and combined, can help in predicting posttransplant prognosis.
Keywords: Hepatocellular carcinoma, Immune checkpoint, Prognosis, Recurrence, Tumor biology
INTRODUCTION
Hepatocellular carcinoma (HCC) is an indication for liver transplantation (LT), especially for living donor LT (LDLT). However, HCC recurrence following LT often results in inferior outcomes. Thus, patients who are candidates for LT have been carefully selected to minimize the risk of posttransplant tumor recurrence. Various selection criteria of LT for HCC have been proposed [1,2,3,4,5,6,7], but most of them have yes-or-no dual concepts, placing many LT candidates in the gray zone for each selection criterion. Additional prognostic biomarkers are therefore necessary to reliably predict posttransplant tumor recurrence. We developed a quantifiable prognostic prediction model of an integrated scoring system derived by multiplying α-FP concentration, concentration of des-γ-carboxyprothrombin level (DCP) or proteins induced by vitamin K antagonists or absence-II (PIVKA-II), and tumor volume (TV), yielding the α-FP-DCP-TV or simply ADV, score [5,6,7,8,9,10].
Cancer immune suppression and immune escape play essential roles in tumor progression. Activation of the programmed death protein 1 (PD-1) pathway is involved in tumor evasion, inhibiting T-cell proliferation, inducing T-cell exhaustion, and enhancing the activity of regulatory T cells [11]. Two types of PD-1 have been identified, membrane-bound PD-1 and soluble PD-1 (sPD-1). Most studies of PD-1 have involved membrane-bound PD-1 in tumor tissue, with fewer studies investigating the role of sPD-1 in peripheral blood. The prognostic impact of sPD-1 concentration in HCC remains unclear to date [12,13,14], although we previously reported that sPD-1 did not have a significant prognostic impact in HCC patients undergoing LT [15].
Preoperative or pretransplant treatment for HCC can alter tumor biology, affecting patient prognosis following hepatic resection or LT. It is therefore necessary to evaluate the tumor biology in patients with untreated HCC. The present study therefore analyzed the prognostic impact of pretransplant sPD-1 concentration and ADV score in patients who underwent LDLT for previously untreated HCC.
METHODS
Study design and patient selection
This retrospective, single-center study assessed posttransplant prognosis in patients with HCC. Its primary purpose was to analyze the risk of posttransplant tumor recurrence in patients with untreated HCC. The secondary purpose was to establish a prognostic prediction model for posttransplant tumor recurrence.
The LT database of the Asan Medical Center was searched to identify adult patients with untreated HCC who underwent LDLT during the 7-year period from January 2010 to December 2016. Patients were excluded if they received any HCC treatment before LT, if HCC was incidentally detected in the explanted livers, if they died within 3 months after LT, or if their peripheral blood samples had not been stored at the Bio-Resource Center of the Asan Medical Center. The 100 patients selected for this study were followed up to determine tumor recurrence and patient survival until August 2020 or patient death through review of institutional medical records and with assistance from the National Health Insurance Service in Korea. The institutional protocols for immunosuppressive regiments, posttransplant follow-up, and treatment of HCC recurrence have been described elsewhere [5,6,7,8,9].
The study protocol was approved by the Institutional Review Board of the Asan Medical Center (No. 2019-0599), which waived the requirement for informed consent due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
Serum sPD-1 assays
sPD-1 concentrations were measured using commercially available ELISA kits (Boster Biological Technology, Pleasanton, CA, USA). Each serum sample was diluted 5-fold with the sample diluent. The quality of sPD-1 was analyzed in test samples, blank samples, and standard controls. Subsequently, 50 µL aliquots of each standard control or sample were added to wells of microtiter plates in triplicate, followed by the addition of 100 µL of horseradish peroxidase-conjugated antibody to each well, except for the blank wells. The wells were covered with adhesive strips and incubated for 1 hour at 37℃. After washing the microtiter plate 5 times, 50 µL of substrate A and 50 µL of substrate B were added to each well. The samples were gently mixed and incubated in the dark for 15 minutes at 37℃. Finally, 50 µL of stop solution was added to each well, and the plates were analyzed spectrophotometrically at 450 nm using a Victor X3 Plate Reader (Bio-Rad Laboratories, Hercules, CA, USA). A standard curve was generated by plotting the average optical density of 450 nm. The details of these assays were described elsewhere [15].
Calculation of ADV scores
ADV scores were calculated by multiplying α-FP (ng/mL) concentration, DCP (mAU/mL) concentration, and TV (mL), expressed on a logarithmic scale (log10, simply log) [5,6,7,8,9,10]. Total TV in patients with multiple tumors was calculated by multiplying the volume of the largest tumor by the number of tumors. α-FP and DCP concentrations were measured within 2 weeks prior to LDLT. Pretransplant and posttransplant ADV scores were calculated using pretransplant imaging study-based TV and pathology report-based TV, respectively.
Statistical analysis
Numerical data were presented as the mean and standard deviation or median and range. Continuous variables were compared using the Student t-test or Mann-Whitney U-test depending on the distribution pattern, and incidence variables were compared using the chi-square test. The cutoffs of sPD-1 concentration and ADV score for predicting posttransplant HCC recurrence were determined using receiver operating characteristic (ROC) curve analysis, with the optimal cutoff, sensitivity, and specificity determined using the Youden index. Tumor recurrence and overall patient survival rates were generated using the Kaplan-Meier method and compared using log-rank tests. Cox proportional hazard regression was used for multivariate analysis, with the results presented as hazard ratio (HR) with 95% confidence interval (CI). A P-value of <0.05 was regarded as statistically significant. All statistical analyses were performed using IBM SPSS ver. 22 (IBM Corp., New York, NY, USA) and MedCalc ver. 20.010 (MedCalc, Ostend, Belgium).
RESULTS
Patient demographics
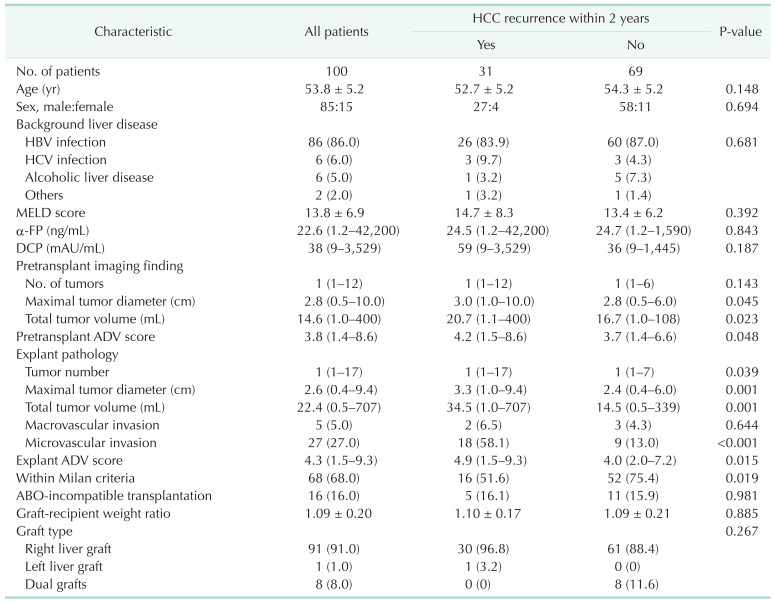
The clinicopathological features of the 100 study patients are summarized in Table 1. The primary diagnoses of the background liver disease included HBV infection in 86 patients, HCV infection in 6, alcoholic liver diseases in 6, and others in 2.
Table 1. Clinicopathological features of the study patients.
Values are presented as number only, mean ± standard deviation, number (%), or median (range).
MELD score, model for end-stage liver disease score; DCP, des-γ-carboxyprothrombin; ADV, α-FP–DCP–tumor volume.
Patients were stratified into 2 groups, based on HCC recurrence within 2 years after LT (Table 1). Groups of patients with and without HCC recurrence within 2 years showed significant differences in pretransplant maximal tumor diameter (P = 0.045), pretransplant total TV (P = 0.023), pretransplant ADV score (P = 0.048), explant tumor number (P = 0.039), explant maximal tumor diameter (P = 0.001), explant total TV (P = 0.001), explant ADV score (P = 0.015), microvascular invasion (P < 0.001), and proportion of patients within the Milan criteria (P = 0.019).
Pretransplant serum sPD-1 concentration and HCC recurrence
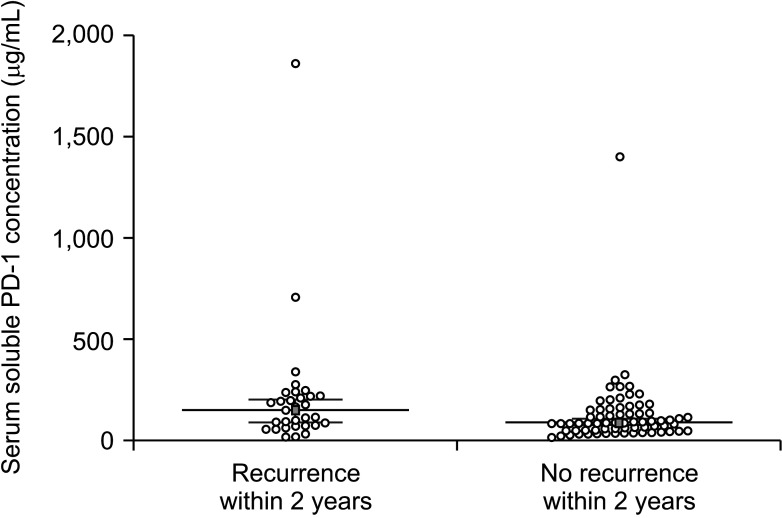
The concentrations of serum sPD-1 are presented in Fig. 1. The mean and median pretransplant serum sPD-1 concentrations were 216.8 ± 332.7 µg/mL and 151.3 µg/mL (range, 18.2–1,864.8 µg/mL), respectively in patients with tumor recurrence within 2 years, and 127.6 ± 172.2 µg/mL and 88.3 µg/mL (range, 15.9–1,404.6 µg/mL) in patients who did not experience tumor recurrence within 2 years (P = 0.028).
Fig. 1. Expression of pretransplant serum soluble programmed death protein 1 (sPD-1) relative to tumor recurrence status at 2 years. The long horizontal bar indicates the median sPD-1 concentration.
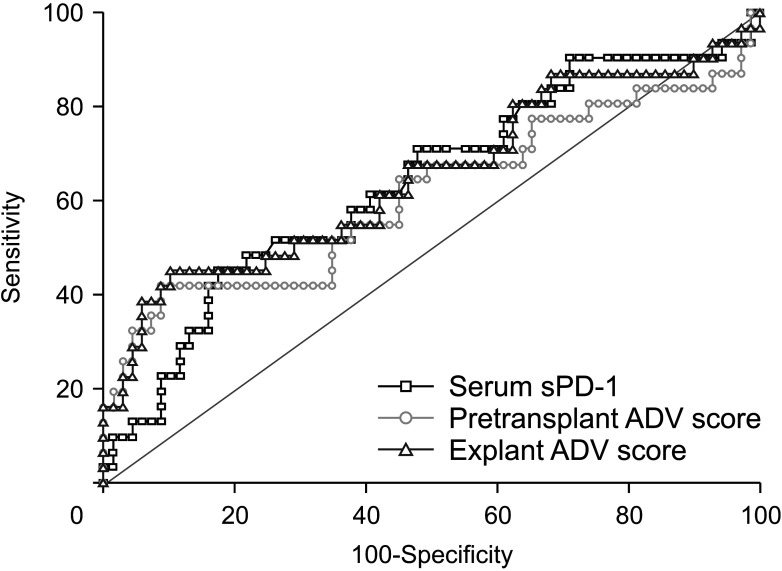
ROC curve analysis of serum sPD-1 concentration for 2-year tumor recurrence showed that the area under the curve (AUC) was 0.637 (95% CI, 0.535–0.731; P = 0.028) (Fig. 2). The Youden index J was 0.278 (95% CI, 0.116–0.418) at an sPD-1 cutoff of 177.1 µg/mL, which showed a sensitivity of 45.2% and a specificity of 82.6%.
Fig. 2. Receiver operating characteristic curve analysis of the effects of soluble programmed death protein 1 (sPD-1) concentration and α-FP–des-γ-carboxyprothrombin–tumor volume (ADV) score on hepatocellular carcinoma recurrence at 2 years.
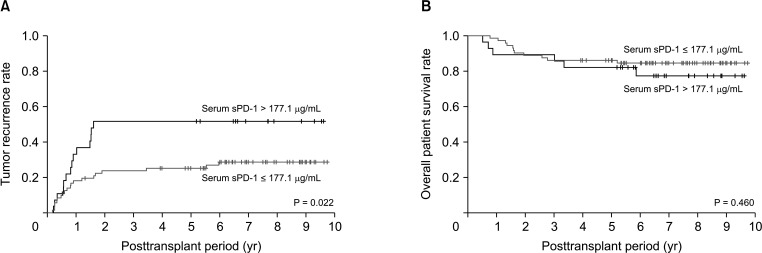
Tumor recurrence rates were significantly higher in the 28 patients with serum sPD-1 of >177.1 µg/mL than in the 72 patients with serum sPD-1 of ≤177.1 µg/mL (P = 0.022) (Fig. 3A). By contrast, overall patient survival rates did not differ significantly in these 2 groups of patients (P = 0.460) (Fig. 3B).
Fig. 3. Kaplan-Meier analysis of (A) tumor recurrence and (B) and overall patient survival rates in patients with pretransplant serum soluble programmed death protein 1 (sPD-1) concentrations of ≤177.1 µg/mL and >177.1 µg/mL.
Pretransplant ADV score and HCC recurrence
ROC curve analysis of pretransplant ADV score for 2-year tumor recurrence showed that the AUC was 0.613 (95% CI, 0.510–0.709; P = 0.102) (Fig. 2). The Youden index J was 0.332 (95% CI, 0.189–0.521) at an ADV score cutoff of 5.4log, which showed a sensitivity of 41.9% and a specificity of 91.3%.
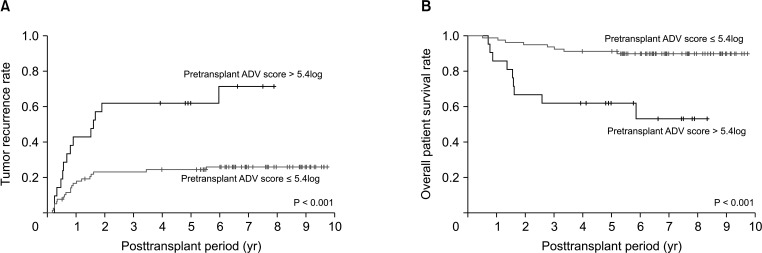
Tumor recurrence rates were significantly higher (P < 0.001) and overall patient survival rates were significantly lower (P < 0.001) in the 21 patients with pretransplant ADV score of >5.4log than in the 79 patients with pretransplant ADV score of ≤5.4log (Fig. 4).
Fig. 4. Kaplan-Meier analysis of (A) tumor recurrence and (B) overall patient survival rates in patients with pretransplant α-FP–des-γ-carboxyprothrombin–tumor volume (ADV) scores of ≤5.4log and >5.4log.
Explant ADV score and HCC recurrence
ROC curve analysis of explant ADV score for 2-year tumor recurrence showed that the AUC was 0.652 (95% CI, 0.550–0.745; P = 0.020) (Fig. 2). The Youden index J was 0.350 (95% CI, 0.185–0.526) at an ADV score cutoff of 5.9log, which showed a sensitivity of 45.2% and a specificity of 89.9%.
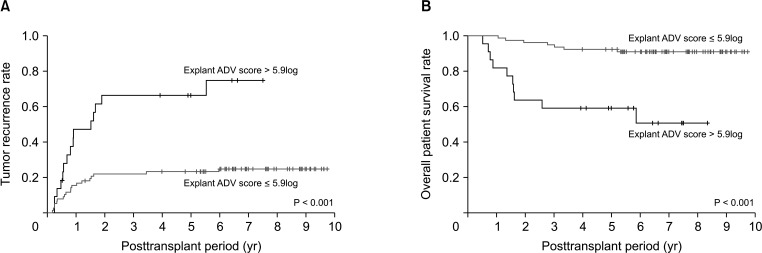
Tumor recurrence rates were significantly higher (P < 0.001) and patient overall survival rates significantly lower (P < 0.001) in the 22 patients with explant ADV score of >5.9log than in the 78 patients with explant A DV score of ≤5.9log (n = 78, 78.0%) (Fig. 5).
Fig. 5. Kaplan-Meier analysis of (A) tumor recurrence and (B) overall patient survival rates in patients with explant α-FP–des-γ-carboxyprothrombin–tumor volume (ADV) scores of ≤5.9log and >5.9log.
Prognostic prediction using a combination of serum sPD-1 and pretransplant ADV score
There was a close correlation between pretransplant and explant ADV scores (Spearman rho = 0.974; 95% CI, 0.922–0.964), and these 2 ADV scores were closely associated with both posttransplant tumor recurrence and overall patient survival. We, therefore, selected pretransplant ADV score for inclusion in a prognostic prediction model for posttransplant HCC recurrence.
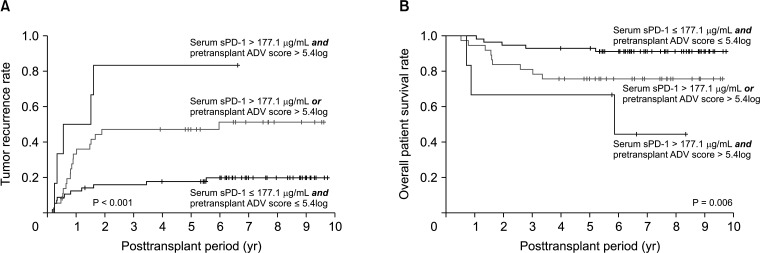
Because sPD-1 of >177.1 µg/mL and pretransplant ADV score of >5.4log were independent risk factors for posttransplant HCC recurrence (Table 2), these factors were combined to stratify patients into 3 groups based on the number of risk factors. The 5-year tumor recurrence rates in subgroups with 0, 1, and 2 risk factors were 17.6%, 47.1%, and 85.3%, respectively (P < 0.001) (Fig. 6A). The 5-year overall patient survival rates in subgroups with 0, 1, and 2 risk factors were 91.2%, 75.7%, and 66.7%, respectively (P = 0.006) (Fig. 6B).
Table 2. Univariate and multivariate analyses on factors associated with posttransplant outcomes.
HR, hazard ratio; CI, confidence interval; PD-1, programmed death protein 1; ADV, α-FP–des-γ-carboxyprothrombin–tumor volume.
Fig. 6. Kaplan-Meier analysis of (A) tumor recurrence and (B) overall patient survival rates in patients with 0, 1, and 2 risk factors for hepatocellular carcinoma recurrence based on a pretransplant serum soluble programmed death protein 1 (sPD-1) concentration cutoff of 177.1 µg/mL and a pretransplant α-FP–des-γ-carboxyprothrombin–tumor volume (ADV) score cutoff of 5.4log.
DISCUSSION
Reliable prediction of posttransplant prognosis in patients who underwent LT for HCC is difficult because tumor burden at the time of LT and tumor biology vary widely and because LT recipients receive lifelong immunosuppression. To avoid HCC recurrence following LT, various patient selection criteria have been proposed [1,2,3,4,5,6,7,16]. These criteria are usually based on radiologic findings, such as tumor size and number, with some of these criteria including biological components such as α-FP and PIVKA-II concentrations. ADV score integrates the tumor size, tumor number, and α-FP and PIVKA-II concentrations for quantitative prediction of HCC recurrence [5].
However, the predictive power of currently available selection criteria is often limited because most of these criteria do not include biologic components of HCC. In the present study, serum sPD-1 concentration served as the biologic component of tumor. However, our previous study in 229 patients who underwent LT found that sPD-1 concentration did not demonstrate a prognostic impact [15]. In that study, 67.2% of study patients had undergone treatment for HCC before LT and only 32.8% had not received treatment for HCC prior to LT [15]. Because differences in tumor responses to treatment may have diluted the prognostic impact of sPD-1, the present study included only patients who had not received treatment for HCC prior to LT.
Cancer immune suppression and immune escape may play critical roles in the progression of malignant tumors. Activation of the PD-1 pathway is involved in tumor evasion, inhibiting T-cell proliferation, inducing T-cell exhaustion, and enhancing the activity of regulatory T cells [11]. During the process of HCC tumorigenesis, the immune surveillance mechanism is impaired by involvement of the PD-1/programmed death-ligand 1 (PD-L1) signaling pathways [16]. Expression of PD-1 in CD8+ T cells is higher in patients with than without HCC [17], with aggressive tumor progression being related to the high frequencies of both circulating and tumor-infiltrating PD-1+ CD8+ T cells [18]. High PD-1 expression in HCC tissue has been associated with aggressive tumor features, with PD-1 expression in HCC tissue closely correlating with the number of CD8+ T cells in peripheral blood [19]. PD-1 and PD-L1 mediate immunosuppression within the tumor microenvironment, suggesting that the levels of PD-1/PD-L1 expression may act as biomarkers for disease progression and patient survival [20].
Immunoregulatory pathways include costimulatory molecules present in both membrane-bound and soluble forms. Proteolytic cleavage of the membrane-bound form of a costimulatory protein in tissues releases the soluble form into the bloodstream. The soluble forms of PD-1/PD-L1 are produced through this process. The associations between PD-1/PD-L1 expression and postresection prognosis of patients with HCC remain unclear [12,13,14]. The association between sPD-1 expression and prognosis in patients with HCC also remains unclear because few studies have assessed the correlations [12,13,14].
No reliable prognostic cutoff of serum sPD-1 for HCC has been determined to date. Our previous study found that an sPD-1 cutoff of 300 µg/mL had a marginal impact on posttransplant tumor recurrence, whereas a cutoff of 93.6 µg/mL was not prognostic [15]. By contrast, the present study revealed that a serum sPD-1 cutoff of 177.1 µg/mL had a significant prognostic impact on posttransplant tumor recurrence. These 2 studies differed in that the present studies excluded patients previously treated for HCCs, had a slightly longer follow-up period, and included an additional number of patients with untreated HCCs. Thus, taken together, these studies suggest that HCC biology associated with sPD-1 expression is greatly influenced by responses to treatment. To our knowledge, this is the first study to show that pretransplant serum sPD-1 concentration is closely associated with HCC recurrence following LT.
ADV score is a quantitative prognostic parameter reflecting the tumor biology of HCC, with prognostic impact in patients who underwent hepatic resection or LT [5,6,7,8,9,10,21]. In the present study, a pretransplant ADV score cutoff of 5.4log was a reliable predictor of posttransplant HCC recurrence and overall patient survival.
Because multivariate analyses revealed that sPD-1 of >177.1 µg/mL and pretransplant ADV score of >5.4log were independent prognostic factors for HCC recurrence, we combined these 2 factors to create a new prognostic model. Patients with both these factors considered high-risk, with a 5-year tumor recurrence rate of 85.3% and a 5-year overall survival rate of 66.7%. Patients with only one of these factors were considered medium-risk with a 5-year tumor recurrence rate of 47.1% and a 5-year overall survival rate of 75.7%. Patients lacking both of these factors were considered low-risk with a 5-year tumor recurrence rate of 17.6% and a 5-year overall survival rate of 91.2%. Because these 2 parameters can be evaluated before LT, this combined risk model may be useful in identifying LT candidates at higher risk and lower risk of posttransplant HCC recurrence.
The present study had several limitations. This was a retrospective, single-center study with a relatively small sample size. Most HCCs developed in HBV-infected livers. The results of the present study were valid only in patients untreated for HCCs, not in those treated for HCC before LT.
In conclusion, the results of the present study revealed that both pretransplant sPD-1 concentration and ADV score have prognostic impact in patients who underwent LT for untreated HCCs. Therefore, each of these parameters as well as their combination can be helpful in predicting posttransplant prognosis. Additional high-volume, multicenter studies are necessary to validate the role of serum sPD-1 in LT recipients.
Footnotes
Fund/Grant Support: This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (grant No. 2021R1A2C2009980 to Shin Hwang).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization, Visualization: SH.
- Data Curation: SH, DBM, GWS, SL, DHJ.
- Formal Analysis: SH, KJL.
- Funding Acquisition: SH.
- Methodology: KJL, YKK, HY, DEA.
- Project Administration: SH, SGL.
- Writing — Original Draft: SH.
- Writing — Review & Editing: All authors.
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 3.Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocelular carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Hwang S, Song GW, Ahn CS, Kim KH, Moon DB, Ha TY, et al. Quantitative prognostic prediction using ADV score for hepatocellular carcinoma following living donor liver transplantation. J Gastrointest Surg. 2021;25:2503–2515. doi: 10.1007/s11605-021-04939-w. [DOI] [PubMed] [Google Scholar]
- 6.Hwang S, Song GW, Ahn CS, Kim KH, Moon DB, Ha TY, et al. Salvage living donor liver transplantation for hepatocellular carcinoma recurrence after hepatectomy: quantitative prediction using ADV score. J Hepatobiliary Pancreat Sci. 2021;28:1000–1013. doi: 10.1002/jhbp.863. [DOI] [PubMed] [Google Scholar]
- 7.Jung DH, Hwang S, Song GW. Selection criteria of living donor liver transplantation for hepatocellular c arc inoma developed in Korean transplant centers. Ann Liver Transplant. 2021;1:29–47. [Google Scholar]
- 8.Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Multiplication of tumor volume by two tumor markers is a post-resection prognostic predictor for solitary hepatocellular carcinoma. J Gastrointest Surg. 2016;20:1807–1820. doi: 10.1007/s11605-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 9.Jung DH, Hwang S, Lee YJ, Kim KH, Song GW, Ahn CS, et al. Small hepatocellular carcinoma with low tumor marker expression benefits more from anatomical resection than tumors with aggressive biology. Ann Surg. 2019;269:511–519. doi: 10.1097/SLA.0000000000002486. [DOI] [PubMed] [Google Scholar]
- 10.Park GC, Hwang S, Park YH, Choi JU Korean Liver Cancer Study Group. Validation of prognostic impact of ADV score for resection of hepatocellular carcinoma: analysis using Korea Liver Cancer Registry Database. Ann Surg Treat Res. 2020;98:235–246. doi: 10.4174/astr.2020.98.5.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang B, Huang T, Wei H, Shen L, Zhu D, He W, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:353–363. doi: 10.1007/s00262-018-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 14.Chang B, Shen L, Wang K, Jin J, Huang T, Chen Q, et al. High number of PD-1 positive intratumoural lymphocytes predicts survival benefit of cytokine-induced killer cells for hepatocellular carcinoma patients. Liver Int. 2018;38:1449–1458. doi: 10.1111/liv.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Na BG, Kim YK, Hwang S, Lee KJ, Park GC, Ahn CS, et al. Absence of association between pretransplant serum soluble programmed death protein-1 level and prognosis following living donor liver transplantation in patients with hepatocellular carcinoma. Medicine (Baltimore) 2021;100:e25640. doi: 10.1097/MD.0000000000025640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha R, Prithviraj P, Anaka M, Bridle KR, Crawford DH, Dhungel B, et al. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front Oncol. 2018;8:269. doi: 10.3389/fonc.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322–3329. doi: 10.3748/wjg.v17.i28.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 19.Kim HD, Song GW, Park S, Jung MK, Kim MH, Kang HJ, et al. Association between expression level of PD1 by tumor-infiltrating CD8+ T cells and features of hepatocellular carcinoma. Gastroenterology. 2018;155:1936–1950. doi: 10.1053/j.gastro.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Moris D, Rahnemai-Azar AA, Zhang X, Ntanasis-Stathopoulos I, Tsilimigras DI, Chakedis J, et al. Program death-1 immune checkpoint and tumor microenvironment in malignant liver tumors. Surg Oncol. 2017;26:423–430. doi: 10.1016/j.suronc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Hwang S, Moon DB, Kim KH, Ahn CS, Song GW, Jung DH, et al. Prognostic accuracy of the ADV score following resection of hepatocellular carcinoma with portal vein tumor thrombosis. J Gastrointest Surg. 2021;25:1745–1759. doi: 10.1007/s11605-020-04800-6. [DOI] [PubMed] [Google Scholar]