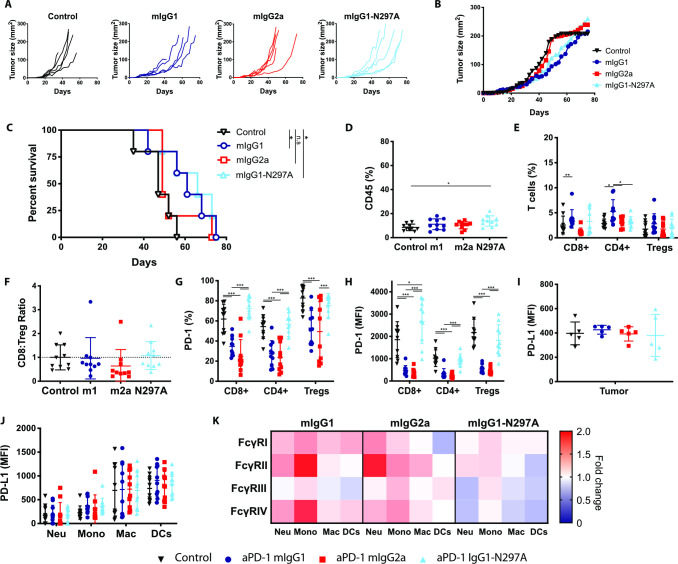
Figure 6.
Similar Fc requirements for anti-PD-1 mAb therapy in cold tumors are not accompanied by improved long-term survival. C57BL/6 mice received 5×105 9464D cells s.c. on day 0. (A–C) When tumors became 5×5 mm mice received weekly doses of 200 µg (i.p) anti-PD-1 isotypes or irrelevant mAbs. Tumor growth was monitored and mice culled when mean tumor area exceeded 225 mm2. Data are presented as tumor area (mm2) for each individual mouse (A) or the mean of the group (B). (C) Kaplan-Meier curves showing percentage survival to humane end point on days after tumor inoculation. Experiment performed once, N=5 mice per group. Log-rank (Mantel-Cox) Test, *p<0.05. (D–K) When tumors became 7×7 mm, mice received three doses of 200 µg (i.p) anti-PD-1 mIgG1, mIgG2a, mIgG1-N297A or irrelevant mAbs on days 1, 5 and 8. Mice were sacrificed on day 9 and spleen and tumor analyzed by flow cytometry. (D, E) Frequency of CD45+ immune infiltrates (D) and T lymphocyte populations (expressed as % of CD45+ cells) (E). F) CD8:Treg ratios expressed as fold change compared with control mice. (G, H) Expression of PD-1 on T lymphocyte populations as % (G) or MFI (H). I–J) Expression of PD-L1 on tumor cells (I) or myeloid infiltrating subpopulations (J) presented as MFI. (K) Heat map indicating relative expression of FcγRs in treatment groups compared with controls. Colors represent the mean ratio of a group, where 1=no change; 1<downregulation; and 1>upregulation relative to controls. Experiment performed twice, n=10 mice per group. Bars represent mean±SD, *p<0.05, **p<0.01, ***p<0.001 (One-way ANOVA). ANOVA, analysis of variance; i.p, intraperitoneally; mAb, monoclonal antibodie; MFI, mean fluorescence intensity; ns, not significant; PD-1, programmed cell-death; s.c., subcutaneously.