Abstract
The multiple breath washout (MBW) test measures the efficiency of gas mixing in the lungs and has gained significant interest over the past 20 years. MBW outcomes detect early lung function impairment and peripheral airway pathology, through its main outcome measure lung clearance index (LCI). LCI measures the number of lung turnovers required to washout an inert tracer gas. MBW is performed during normal (tidal) breathing, making it particularly suitable for young children or those who have trouble performing forced manoeuvres. Additionally, research in chronic respiratory disease populations has shown that MBW can detect acute clinically relevant changes before conventional lung function tests, such as spirometry, thus enabling early intervention. The development of technical standards for MBW and commercial devices have allowed MBW to be implemented in clinical research and potentially routine clinical practice. Although studies have summarised clinimetric properties of MBW indices, additional research is required to establish the clinical utility of MBW and, if possible, shorten testing time. Sensitive, feasible measures of early lung function decline will play an important role in early intervention for people living with respiratory diseases.
Educational aim
To describe the multiple breath washout test, its applications to lung pathology and respiratory disease, as well as directions for future research.
Short abstract
The multiple breath washout test is a sensitive measure of early lung function impairment. It has been shown to be feasible in young children and several respiratory disease populations; nonetheless more work is required to establish its clinical utility. https://bit.ly/2W5xiol
Introduction
Many common obstructive pulmonary diseases originate in the peripheral airways, often manifesting long before symptoms appear or are detected by standard pulmonary function tests (e.g. spirometry). The most commonly used spirometric indices, such as forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), predominately reflect large airway function and therefore miss the opportunity for early detection of damage to the small airways and intervention to prevent long term sequelae [1–3]. While forced expiratory flows (i.e. forced expiratory flow at 25–75% of FVC) may be more sensitive to small airway changes than FEV1 or FVC and can detect differences between groups, they are highly variable and influenced by FVC which makes them difficult to interpret in an individual [4, 5]. The multiple breath washout (MBW) test offers the ability to detect early manifestations of peripheral airway pathology by assessing gas mixing within the lungs, a process that all airways participate in [6–8]. The MBW test was first described in 1952 by Dr Margaret Becklake [9]; however, interest in the technique has only gained significant traction in the past 20 years. Consensus recommendations regarding technical standards for equipment and measurement protocols have been integral to improving the accuracy and consistency of results and the subsequent emergence of robust commercial devices [10]. Validation of MBW equipment is recommended, and the in vitro precision of measured functional residual capacity (FRC) for several devices using a double chamber plastic lung model is within 5% across a range of lung volumes and respiratory frequencies [11–14]. The availability of commercial devices has helped facilitate the implementation of MBW into multicentre international research studies [15, 16], and its transition into routine clinical practice [17]. In this review we describe the MBW test, its applications and challenges, and directions for future research.
What is multiple breath washout?
MBW assesses ventilation distribution by measuring how efficiently the lungs clear an inert tracer gas across a series of breaths (figure 1). The lungs have evolved to promote efficient gas mixing, and in healthy individuals, an inhaled gas should distribute evenly throughout the lungs. The distribution of obstructive lung disease is typically patchy in its early stages, and this narrowing/obstruction of peripheral airways leads to uneven and less efficient gas mixing. Therefore, the longer it takes to wash out the tracer gas of interest, the greater the ventilation inhomogeneity present and the less efficient gas mixing is. The “inert” tracer gas must be safe to inhale and not participate in gas exchange. Exogenous gases (e.g. sulfur hexafluoride (SF6) and helium) must be washed-in before they are washed-out by breathing room air, whereas endogenous gases (e.g. nitrogen) are washed-out by breathing 100% oxygen (figure 1). One of the advantages of MBW is that it can be performed during tidal (normal) breathing, which makes it ideally suited for infants and young children, as well as some adults, who have trouble performing the forced manoeuvres required for standard pulmonary function tests [17, 18].
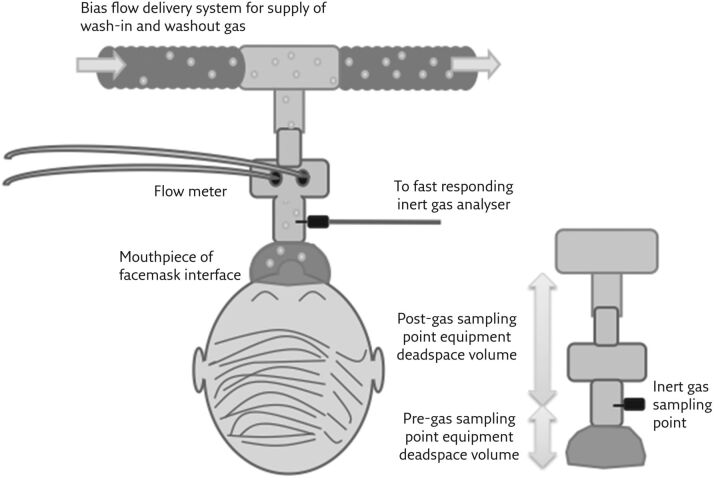
Figure 1.
Schematic illustration of a generic inert gas washout system. Hardware required for washout is relatively simple: a flow meter, a fast-responding inert gas analyser, a gas delivery system, and a patient interface. The equipment-related deadspace volume (VD) can be divided into pre- and post-gas sampling points. Post-gas sampling point VD effectively introduces a small rebreathing chamber. Pre-gas sampling point VD is an extension of anatomical VD. Reproduced from [10] with permission.
The lung clearance index (LCI), the most commonly reported outcome measure from the MBW test, is a global measure of ventilation inhomogeneity. It is calculated as the cumulative expired volume of air exhaled during the washout portion of the test, corrected for a measure of the subject’s lung volume (FRC), which is also calculated during the test. In other words, the LCI represents the number of lung volume turnovers (FRCs) required to clear the tracer gas. The change in slope of the normalised nitrogen alveolar plateau (phase III slope) over a series of breaths can also be reported to distinguish ventilation heterogeneity arising within proximal conducting airways (Scond) from that arising in more distal airways within the region of the lung acinus (Sacin) [10, 19].
In research studies the feasibility of MBW is often greater than 80% [20, 21]; whereas when implemented into clinical practice the feasibility drops to 60–70% [8, 22]. The feasibility is also higher in school-age children compared with preschool children; however, with the appropriate training and child-friendly environment, feasibility can be up to 89% in preschool children [23, 24]. The majority of studies, to date, have measured LCI in individuals with normal measures of spirometry, or with mild lung disease. The LCI is more variable in individuals with more advanced lung disease and may not be as useful in those with reduced lung function measured from forced expiratory manoeuvres.
Interpretation of results
As with all pulmonary function tests, MBW must meet the technical requirements of a good quality test before results can be interpreted [10, 25]. In addition, repeatable trials must be obtained to ensure the measured indices reflect the physiology within the lungs; the average of at least 2–3 trials is typically reported. Although some criteria are clear and objective to define, evaluation of the quality of a washout curve requires careful breath-by-breath review. For example, at least three breaths must be present under the target end concentration with no evidence of leaks (i.e. the intake of room air causing a change in the tracer gas concentration measured by the system). In the example depicted in figure 2, the washout curve includes several examples of leaks, and the interpretation of the results produced by this washout would be biased and not reflect the true underlying ventilation inhomogeneity within the lungs.
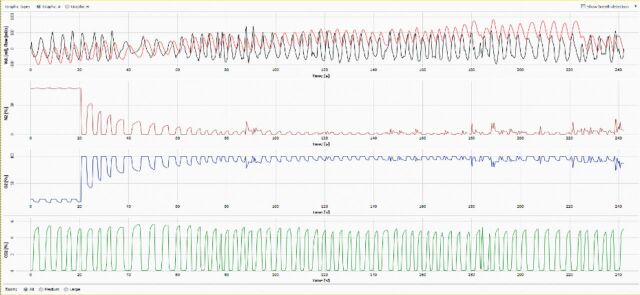
Figure 2.
Example of a washout curve. From top to bottom, the breath-by-breath tracings show the volume/flow (red/black), nitrogen (N2) concentration (red), oxygen (O2) concentration (blue), and carbon dioxide (CO2) concentration (green). In this example there are multiple instances of gas leaks, apparent as increases or spikes in nitrogen concentration, where external nitrogen has been entrained and measured by the system. As the test measures the washout of nitrogen from the lungs, nitrogen contained in room air that enters the system will increase the measured gas concentration and over-estimate the LCI [26].
Furthermore, the breathing pattern should reflect normal tidal breathing. In adult testing, some respiratory function laboratories have used a fixed 1 L tidal volume breathing protocol [27] to ensure clear visualisation of the phase III slope and a more stable breathing pattern. However, in young children this approach is not feasible, and in older children this may lead to significantly different LCI and FRC results [28]. Although extreme deviations from normal tidal breathing may impact LCI and other indices, some degree of variability in breathing pattern is expected and does not necessarily influence outcomes [29]. Further research is needed to identify which quality control criteria impact results significantly. Implementation of clear, objective criteria to evaluate the quality of the washout in real-time will help to increase the confidence with which results can be interpreted and may not require detailed review. These efforts are already well underway [15].
How much change in LCI is important?
As with all pulmonary function tests, it is important to monitor changes over time to understand whether changes in lung function are associated with clinically meaningful events. Quantifying the magnitude of change that can be attributed to measurement error and/or biological variability is necessary to be able to distinguish noise from clinically meaningful changes. For the MBW tests, the measurement error of the test can be minimised by appropriate calibration of equipment and test measurement protocols, whereas the biological variability of the test has been shown to be proportional to the degree of impairment (figure 3). In other words, the variability of LCI is higher in individuals with worse LCI. Initially, evidence suggested a 1-unit change in LCI was considered meaningful, and studies were designed to look at whether clinical care could be guided when a 1-unit change in LCI was observed [30]. However, since variability is proportional to the measured value, a 1-unit change may underestimate variability in those with worse LCI values, and therefore overestimate meaningful changes, whilst missing important changes in those with values closer to the normal range. For example, a 1-unit change in an individual with an LCI of 15 represents a 6.6% change, whereas a 1-unit change in an individual with an LCI of 7 represents a 14% change. Therefore, reporting relative change in LCI may be a solution to this, with changes greater than 15% considered outside variability observed in health and stable cystic fibrosis (CF) [31, 32]. Further studies are needed to verify these cut-off points, or to develop anchor-based approaches that are tied to clinical end-points.
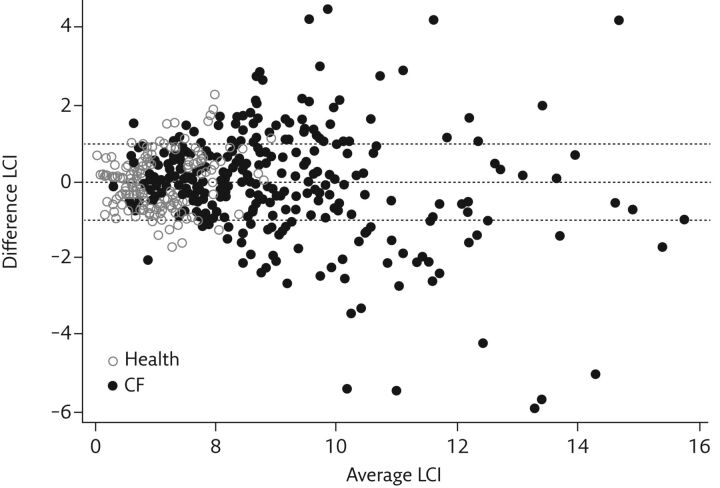
Figure 3.
Bland–Altman plot of the difference in repeated LCI measurements. The difference between LCI measurements is greater at higher LCI values. CF: cystic fibrosis. Reproduced from [31] with permission.
Shortening the testing time?
The biggest barrier to implementation of the MBW into the busier environment of routine clinical care is the time required to complete the test and obtain 2–3 good quality curves. There is strong evidence in support of using earlier cut-offs, namely LCI5 at 1/20th of the starting concentration. The earlier cut-point can save considerable time (∼30–40%) and produces similar results in terms of repeatability, diagnostic characteristics, and detecting significant treatment effects [33–35]. Yet there is some concern that shortening the washout may reduce the sensitivity to detect lung function impairment [36]. LCI5 (i.e. LCI at 1/20th of the starting concentration) has currently not been widely used in clinical care or research and future work is needed to define the clinimetric properties of LCI5 to aid clinical interpretation and implementation.
What's next?
The feasibility for measuring MBW in childhood, including infants and preschool children, has been repeatedly demonstrated for different MBW devices and tracer gases across both the clinical and research environment. Most of the research has focused on the paediatric CF population. As the majority of preschool-age children with CF have an elevated LCI [22, 24, 37], further standardisation efforts to develop robust equipment and define testing protocols for infants are necessary, and currently ongoing [10, 38].
While the vast majority of MBW research to date has been in the CF population, increasingly MBW is also being used in other obstructive lung diseases. For example, MBW outcomes have been used in adults to detect the early damage from cigarette smoking [39], bronchiectasis [16], COPD [40] and early post-transplant bronchiolitis obliterans syndrome (BOS) [41, 42]. Ventilation inhomogeneity is present in asthma (both mild and uncontrolled) [43–45]. More recently a study of symptomatic military deployers was able to detect airway injury due to exposure to high concentrations of particulate matter from sandstorms, diesel combustion, and burning waste using MBW [46]. Cumulatively, these studies highlight the potential for MBW to be used as a screening tool to detect early peripheral airway injury (table 1).
Table 1.
Summary of potential utility across disease groups
| Disease group | Use |
| Healthy | Assess airway function in individuals too young to perform spirometry |
| Asthma | Assess ventilation inhomogeneity Assess asthma control |
| CF | Detect early lung damage Tracking longitudinal changes in lung function Assessing response to treatment |
| Bronchiectasis | Detect early peripheral airway involvement |
| COPD | Detect early peripheral airway involvement |
| BOS | Detection of early post-transplant BOS |
A number of challenges remain before recommendations can be made to use MBW as part of routine clinical care. Although normative reference ranges, as well as within- and between-test reproducibility limits have been published [31, 32, 47–49], the minimal clinically important difference remains to be determined, and we need to better understand how often measurements should be performed, and for which conditions MBW has demonstratable clinical utility. There are several commercial MBW devices now available, each using different gases and/or sensors. Despite standards for equipment and software and validation studies, results from different devices/gases are not interchangeable [50]. Currently, reference equations for healthy populations need to be derived for each device separately. There is a European Respiratory Society task force underway to develop standardised reference equations. The development of these equations, along with further adaptations to software and testing protocols, will aid incorporation into clinical practice.
Conclusions
There is accumulating evidence that the peripheral airways play an important role in several obstructive lung diseases. In addition, the origins of many chronic respiratory diseases lie in early life. Sensitive, feasible measures of lung function to detect peripheral airway changes in childhood and early adulthood, such as MBW, are likely to play an important role in future screening and early intervention strategies.
Key points
The MBW test detects peripheral airway narrowing/obstruction by measuring the efficiency of gas mixing in the lungs.
Standard pulmonary function tests, such as spirometry, predominantly reflect large airway function and therefore are less sensitive to early signs of lung function impairment in the peripheral airways.
MBW has evolved in the past 20 years, and now has validated commercial equipment, detailed technical standards, and established clinimetric properties.
Although the MBW test has been implemented into international clinical trials, further work is required to better understand its clinical utility.
Self-evaluation questions
-
Can you interpret the LCI value from a single MBW trial?
a) Yes, provided it is a good quality trial
b) No, you need to report at least two good trials
c) Yes, as only one trial can be obtained from participants
-
Can you use LCI5 (LCI at 5% of the starting gas concentration) instead of LCI2.5 (LCI at 2.5% of the starting gas concentration) to define increased ventilation inhomogeneity?
a) No, the LCI5 has reduced sensitivity and you may miss important changes
b) Yes, the LCI5 and LCI2.5 have the same diagnostic characteristics
c) Yes, the LCI5 and LCI2.5 are interchangeable and provide the same information
-
Which of the following statements is true?
a) The clinical utility of the MBW test will improve with adaptations to reduce testing time
b) The MBW test can only be used in infants and young children with CF
c) The MBW test requires expensive gases that are difficult to purchase
Suggested answers
b.
a.
a.
Footnotes
Conflict of interest: S. Stanojevic has nothing to disclose.
Conflict of interest: C. Bowerman has nothing to disclose.
Conflict of interest: P. Robinson has nothing to disclose.
References
- 1.Aurora P, Stanojevic S, Wade A, et al. . Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med 2011; 183: 752–758. doi: 10.1164/rccm.200911-1646OC [DOI] [PubMed] [Google Scholar]
- 2.Horsley AR, Gustafsson PM, Macleod KA, et al. . Lung clearance index is a sensitive, repeatable and practical measure of airways disease in adults with cystic fibrosis. Thorax 2008; 63: 135–140. doi: 10.1136/thx.2007.082628 [DOI] [PubMed] [Google Scholar]
- 3.Rowan SA, Bradley JM, Bradbury I, et al. . Lung clearance index is a repeatable and sensitive indicator of radiological changes in bronchiectasis. Am J Respir Crit Care Med 2014; 189: 586–592. doi: 10.1164/rccm.201310-1747OC [DOI] [PubMed] [Google Scholar]
- 4.Pellegrino R, Viegi G, Brusasco V, et al. . Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 5.Quanjer PH, Weiner DJ, Pretto JJ, et al. . Measurement of FEF25–75% and FEF75% does not contribute to clinical decision making. Eur Respir J 2014; 43: 1051–1058. doi: 10.1183/09031936.00128113 [DOI] [PubMed] [Google Scholar]
- 6.Lum S, Gustafsson P, Ljungberg H, et al. . Early detection of cystic fibrosis lung disease: multiple-breath washout versus raised volume tests. Thorax 2007; 62: 341–347. doi: 10.1136/thx.2006.068262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafsson PM, Aurora P, Lindblad A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J 2003; 22: 972–979. doi: 10.1183/09031936.03.00049502 [DOI] [PubMed] [Google Scholar]
- 8.Stahl M, Joachim C, Blessing K, et al. . Multiple breath washout is feasible in the clinical setting and detects abnormal lung function in infants and young children with cystic fibrosis. Respiration 2014; 87: 357–363. doi: 10.1159/000357075 [DOI] [PubMed] [Google Scholar]
- 9.Becklake MR. A new index of the intrapulmonary mixture of inspired air. Thorax 1952; 7: 111–116. doi: 10.1136/thx.7.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson PD, Latzin P, Verbanck S, et al. . Consensus statement for inert gas washout measurement using multiple- and single-breath tests. Eur Respir J 2013; 41: 507–522. doi: 10.1183/09031936.00069712 [DOI] [PubMed] [Google Scholar]
- 11.Fuchs SI, Buess C, Lum S, et al. . Multiple breath washout with a sidestream ultrasonic flow sensor and mass spectrometry: a comparative study. Pediatr Pulmonol 2006; 41: 1218–1225. doi: 10.1002/ppul.20524 [DOI] [PubMed] [Google Scholar]
- 12.Singer F, Houltz B, Latzin P, et al. . A realistic validation study of a new nitrogen multiple-breath washout system. PLoS One 2012; 7: e36083. doi: 10.1371/journal.pone.0036083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt A, Yammine S, Proietti E, et al. . Validation of multiple-breath washout equipment for infants and young children. Pediatr Pulmonol 2015; 50: 607–614. doi: 10.1002/ppul.23010 [DOI] [PubMed] [Google Scholar]
- 14.Singer F, Houltz B, Robinson P, et al. . Bench test of a mass spectrometer based multiple-breath washout system using a realistic lung model. Eur Respir J 2012; 40: Suppl. 56, P4602. [Google Scholar]
- 15.Saunders C, Jensen R, Robinson PD, et al. . Integrating the multiple breath washout test into international multicentre trials. J Cyst Fibros 2020; 19: 602–607. doi: 10.1016/j.jcf.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 16.O'Neill K, Ferguson K, Cosgrove D, et al. . Multiple breath washout in bronchiectasis clinical trials: is it feasible? ERJ Open Res 2020; 6: 00363-2019. doi: 10.1183/23120541.00363-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer F, Kieninger E, Abbas C, et al. . Practicability of nitrogen multiple-breath washout measurements in a pediatric cystic fibrosis outpatient setting. Pediatr Pulmonol 2013; 48: 739–746. doi: 10.1002/ppul.22651 [DOI] [PubMed] [Google Scholar]
- 18.Aurora P, Bush A, Gustafsson P, et al. . Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med 2005; 171: 249–256. doi: 10.1164/rccm.200407-895OC [DOI] [PubMed] [Google Scholar]
- 19.Verbanck S, Schuermans D, Van Muylem A, et al. . Conductive and acinar lung-zone contributions to ventilation inhomogeneity in COPD. Am J Respir Crit Care Med 1998; 157: 1573–1577. doi: 10.1164/ajrccm.157.5.9710042 [DOI] [PubMed] [Google Scholar]
- 20.Robinson PD, Latzin P, Ramsey KA, et al. . Preschool multiple-breath washout testing an official American thoracic society technical statement. Am J Respir Crit Care Med 2018; 197: e1–e19. doi: 10.1164/rccm.201801-0074ST [DOI] [PubMed] [Google Scholar]
- 21.Stanojevic S, Davis SD, Perrem L, et al. . Determinants of lung function progression measured by lung clearance index in children with cystic fibrosis. Eur Respir J 2021; 58: 2003380. doi: 10.1183/13993003.03380-2020 [DOI] [PubMed] [Google Scholar]
- 22.Hardaker KM, Panda H, Hulme K, et al. . Abnormal preschool Lung Clearance Index (LCI) reflects clinical status and predicts lower spirometry later in childhood in cystic fibrosis. J Cyst Fibros 2019; 18: 721–727. doi: 10.1016/j.jcf.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Ratjen F, Davis SD, Stanojevic S, et al. . Inhaled hypertonic saline in preschool children with cystic fibrosis (SHIP): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2019; 7: 802–809. doi: 10.1016/S2213-2600(19)30187-0 [DOI] [PubMed] [Google Scholar]
- 24.Stanojevic S, Davis SD, Retsch-Bogart G, et al. . Progression of lung disease in preschool patients with cystic fibrosis. Am J Respir Crit Care Med 2017; 195: 1216–1225. doi: 10.1164/rccm.201610-2158OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen R, Stanojevic S, Klingel M, et al. . A systematic approach to multiple breath nitrogen washout test quality. PLoS One 2016; 11: e0157523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenherr N, Ramsey KA, Jost K, et al. . Leaks during multiple-breath washout: characterisation and influence on outcomes. ERJ Open Res 2018; 4: 00012-2017. doi: 10.1183/23120541.00012-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verbanck S, Schuermans D, Van Muylem A, et al. . Ventilation distribution during histamine provocation. J Appl Physiol 1997; 83: 1907–1916. doi: 10.1152/jappl.1997.83.6.1907 [DOI] [PubMed] [Google Scholar]
- 28.Yammine S, Singer F, Gustafsson P, et al. . Impact of different breathing protocols on multiple-breath washout outcomes in children. J Cyst Fibros Elsevier 2014; 13: 190–197. doi: 10.1016/j.jcf.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 29.Ratjen F, Jensen R, Klingel M, et al. . Effect of changes in tidal volume on multiple breath washout outcomes. PLoS One 2019; 14: e0219309. doi: 10.1371/journal.pone.0219309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voldby C, Green K, Kongstad T, et al. . Lung clearance indextriggered intervention in children with cystic fibrosis A randomised pilot study. J Cyst Fibros 2020; 19: 934–941. doi: 10.1016/j.jcf.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 31.Engberink E Oude, Ratjen F, Davis SD, et al. . Inter-test reproducibility of the lung clearance index measured by multiple breath washout. Eur Respir J 2017; 50: 1700433. doi: 10.1183/13993003.00433-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svedberg M, Gustafsson PM, Robinson PD, et al. . Variability of lung clearance index in clinically stable cystic fibrosis lung disease in school age children. J Cyst Fibros 2018; 17: 236–241. doi: 10.1016/j.jcf.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Yammine S, Singer F, Abbas C, et al. . Multiple-breath washout measurements can be significantly shortened in children. Thorax 2013; 68: 586–587. doi: 10.1136/thoraxjnl-2012-202345 [DOI] [PubMed] [Google Scholar]
- 34.Hannon D, Bradley JM, Bradbury I, et al. . Shortened lung clearance index is a repeatable and sensitive test in children and adults with cystic fibrosis. BMJ Open Respir Res 2014; 1: e000031. doi: 10.1136/bmjresp-2014-000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw M, Oppelaar MC, Jensen R, et al. . The utility of moment ratios and abbreviated endpoints of the multiple breath washout test in preschool children with cystic fibrosis. Pediatr Pulmonol 2020; 55: 649–653. doi: 10.1002/ppul.24618 [DOI] [PubMed] [Google Scholar]
- 36.Stanojevic S, Jensen R, Sundaralingam D, et al. . Alternative outcomes for the multiple breath washout in children with CF. J Cyst Fibros 2015; 14: 490–496. doi: 10.1016/j.jcf.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 37.Ramsey KA, Rosenow T, Turkovic L, et al. . Lung clearance index and structural lung disease on computed tomography in early cystic fibrosis. Am J Respir Crit Care Med 2016; 193: 60–67. doi: 10.1164/rccm.201507-1409OC [DOI] [PubMed] [Google Scholar]
- 38.Gustafsson PM, Kadar L, Kjellberg S, et al. . End-expiratory lung volume remains stable during N2 MBW in healthy sleeping infants. Physiol Rep 2020; 8: e14477. doi: 10.14814/phy2.14477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jetmalani K, Thamrin C, Farah CS, et al. . Peripheral airway dysfunction and relationship with symptoms in smokers with preserved spirometry. Respirology 2018; 23: 512–518. doi: 10.1111/resp.13215 [DOI] [PubMed] [Google Scholar]
- 40.Zaigham S, Wollmer P, Engström G. The association of lung clearance index with COPD and FEV1 reduction in ‘Men born in 1914’. COPD J Chronic Obstr Pulm Dis 2017; 14: 324–329. doi: 10.1080/15412555.2017.1314455 [DOI] [PubMed] [Google Scholar]
- 41.Thompson BR, Ellis MJ, Stuart-Andrews C, et al. . Early bronchiolitis obliterans syndrome shows an abnormality of perfusion not ventilation in lung transplant recipients. Respir Physiol Neurobiol 2015; 216: 28–34. doi: 10.1016/j.resp.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 42.Driskel M, Horsley A, Fretwell L, et al. . Lung clearance index in detection of post-transplant bronchiolitis obliterans syndrome. ERJ Open Res 2019; 5: 00164–2019. doi: 10.1183/23120541.00164-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuttall AGL, Velásquez W, Beardsmore CS, et al. . Lung clearance index: assessment and utility in children with asthma. Eur Respir Rev 2019; 28: 190046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbanck S, Schuermans D, Noppen M, et al. . Evidence of acinar airway involvement in asthma. Am J Respir Crit Care Med 1999; 159: 1545–1550. doi: 10.1164/ajrccm.159.5.9809017 [DOI] [PubMed] [Google Scholar]
- 45.Kjellberg S, Houltz BK, Zetterström O, et al. . Clinical characteristics of adult asthma associated with small airway dysfunction. Respir Med 2016; 117: 92–102. doi: 10.1016/j.rmed.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 46.Zell-Baran LM, Krefft SD, Moore CM, et al. . Multiple breath washout: a noninvasive tool for identifying lung disease in symptomatic military deployers. Respir Med 2020; 176: 106281. doi: 10.1016/j.rmed.2020.106281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green K, Kongstad T, Skov M, et al. . Variability of monthly nitrogen multiple-breath washout during one year in children with cystic fibrosis. J Cyst Fibros 2018; 17: 242–248. doi: 10.1016/j.jcf.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 48.Anagnostopoulou P, Latzin P, Jensen R, et al. . Normative data for multiple breath washout outcomes in school-aged Caucasian children. Eur Respir J 2020; 55: 1901302. doi: 10.1183/13993003.01302-2019 [DOI] [PubMed] [Google Scholar]
- 49.Verbanck S, Van Muylem A, Schuermans D, et al. . Transfer factor, lung volumes, resistance and ventilation distribution in healthy adults. Eur Respir J 2016; 47: 166–176. doi: 10.1183/13993003.00695-2015 [DOI] [PubMed] [Google Scholar]
- 50.Bayfield KJ, Horsley A, Alton E, et al. . Simultaneous sulfur hexafluoride and nitrogen multiple-breath washout (MBW) to examine inherent differences in MBW outcomes. ERJ Open Res 2019; 5: 00234–2018. doi: 10.1183/23120541.00234-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]