Abstract
OBJECTIVE
Achieving optimal glycemic control for many individuals with type 1 diabetes (T1D) remains challenging, even with the advent of newer management tools, including continuous glucose monitoring (CGM). Modern management of T1D generates a wealth of data; however, use of these data to optimize glycemic control remains limited. We evaluated the impact of a CGM-based decision support system (DSS) in patients with T1D using multiple daily injections (MDI).
RESEARCH DESIGN AND METHODS
The studied DSS included real-time dosing advice and retrospective therapy optimization. Adults and adolescents (age >15 years) with T1D using MDI were enrolled at three sites in a 14-week randomized controlled trial of MDI + CGM + DSS versus MDI + CGM. All participants (N = 80) used degludec basal insulin and Dexcom G5 CGM. CGM-based and patient-reported outcomes were analyzed. Within the DSS group, ad hoc analysis further contrasted active versus nonactive DSS users.
RESULTS
No significant differences were detected between experimental and control groups (e.g., time in range [TIR] +3.3% with CGM vs. +4.4% with DSS). Participants in both groups reported lower HbA1c (−0.3%; P = 0.001) with respect to baseline. While TIR may have improved in both groups, it was statistically significant only for DSS; the same was apparent for time spent <60 mg/dL. Active versus nonactive DSS users showed lower risk of and exposure to hypoglycemia with system use.
CONCLUSIONS
Our DSS seems to be a feasible option for individuals using MDI, although the glycemic benefits associated with use need to be further investigated. System design, therapy requirements, and target population should be further refined prior to use in clinical care.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease that results in complete insulin deficiency (1). The only known effective treatment available for this condition is insulin replacement through daily injections or insulin pumps. Currently, 1.6 million people have been diagnosed with T1D in the U.S. (2), and between 30% and 60% of them are using insulin pumps (3–7). Continuous subcutaneous insulin infusion (CSII) is considered the gold standard of intensive insulin treatment, especially if used in conjunction with continuous glucose monitoring (CGM) and an automated insulin delivery (AID) system (1). Insulin pumps are able to mimic the physiological insulin delivery disrupted by the autoimmune process; however, CSII comes with several challenges that require specific skills from the user and higher levels of diabetes self-care engagement, as compared to insulin delivery through multiple daily injections (MDI). Changing the insulin infusion set, counting carbohydrates, and administering correction boluses are just a few of them (8). Insulin pumps are also visible devices that may create concerns in potential users who prefer to reduce the burden of device wear, maintain a higher level of privacy related to their condition, and lower the possibility of being labeled as diabetic or, more generally, as sick by others (9). A tethered pump that is not waterproof can also be difficult for swimmers and other competitive athletes. Cost can also be a significant barrier preventing people from using insulin pumps (8). Therefore, it is not surprising that a large group of individuals with T1D choose to use MDI as their insulin delivery method.
The focus on AID systems over the past decade has left MDI users largely unable to benefit from the latest technology developments in diabetes care, which for the most part rely on insulin pumps for accurate real-time insulin delivery modulation. Although CGM has been shown to be effective in reducing hypoglycemia and improving time in range (TIR) for MDI users (10), systems capable of filtering the wealth of information generated and providing actionable information to MDI users are still in their infancy (11–13). Diabetes support systems (DSS) can be defined as “the provision of person-specific information, intelligently filtered, prioritized and presented at the right time to patients and clinicians, to enhance health and health care” (14). DSS are becoming increasingly popular in clinical settings as a tool to guide physicians caring for hospitalized individuals with diabetes (15). Although DSS in outpatient settings have been available for almost 40 years, this technology is still rarely used (16). Preliminary studies have proven they can be a beneficial tool capable of reducing glycemic variability and preventing hypoglycemia (17–19), but meta-analyses of a variety of systems suggest that the impact of DSS on the main diabetes outcomes (i.e., HbA1c, TIR between 70 and 180 mg/dL, time above range [TAR], time below range [TBR]) is marginal (16,20).
This study investigated the benefits of a DSS on a relatively large cohort of individuals with T1D who use MDI as their insulin delivery method. The primary outcome of the study was to determine the effect of DSS on TIR, defined as the percentage of CGM values in the 70–180 mg/dL range. We also evaluated the impact of our DSS on the risk of hypoglycemia, glycemic variability, HbA1c, and psychobehavioral markers.
Research Design and Methods
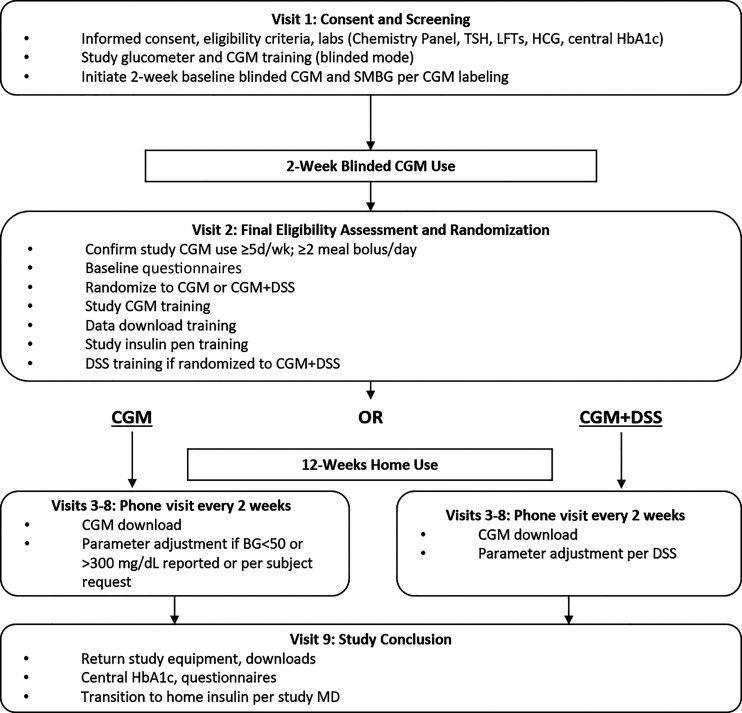
The study was approved by the institutional review board of each site, and an investigational device exemption was obtained from the U.S. Food and Drug Administration (G17033). The study was conducted by three institutions: the University of Virginia Center for Diabetes Technology, Stanford University, and the Icahn School of Medicine at Mount Sinai. Participants were recruited from all sites. Major eligibility criteria included diagnosis of T1D and treated with insulin for at least 1 year, use of basal and meal insulin for intensive insulin therapy (including carbohydrate counting) for at least 1 month, and age ≥15 years. The study was initiated and conducted between June 2017 and March 2019. Following the screening visit, all participants wore a blinded CGM (Dexcom G4; Dexcom, San Diego, CA) during a 2-week assessment phase using their own carbohydrate ratio and correction factor parameters. Following this baseline data collection, participants were randomly assigned at a 2:1 ratio to either the experimental (DSS + MDI + CGM) or control group (MDI + CGM). During the 12-week main protocol phase, all participants used an unblinded CGM (Dexcom G5) and transitioned from their usual basal and mealtime insulins to insulin degludec and insulin aspart, respectively. Participants used reusable smart insulin pens (NovoPen 6 and NovoPen Echo +; Novo Nordisk, Bagsværd, Denmark) with near-field communication connectivity. Participants in the experimental group were asked to use our newly developed DSS deployed on a portable medical application platform (inControl Advice; TypeZero Technologies, Charlottesville, VA). The study design is illustrated in Fig. 1.
Figure 1.
Study flow diagram. BG, blood glucose; HCG, human chorionic gonadotropin; LFT, liver function test; SMBG, self-monitoring of blood glucose; TSH, thyrotropin.
The inControl Advice app included several features and functions: 1) CGM connectivity, data storage, and display; 2) near-field communication pen connectivity and data storage; 3) smart bolus calculator based on CGM values, glycemic prediction, and insulin on board; 4) hypoglycemia detector with accompanying warning system and recommendation for blood glucose monitoring and treatment; 5) long-term average CGM tracker; 6) bedtime button, activated by the user before bedtime, that assessed risk of overnight hypoglycemia and recommended a bedtime snack if hypoglycemia risk was elevated; 7) exercise risk warning system, capable of predicting hypoglycemia at the onset of physical activity and advising on mitigating treatments, such as carbohydrate consumption; and 8) biweekly MDI treatment parameter optimization routine, using 1 month of collected CGM, insulin, and meal data.
In addition, the InControl Advice app was connected with a cloud-based infrastructure, allowing for delocalized computations, system monitoring, over-the-air software updates, and system and device management (Supplementary Fig. 1).
Participants randomly assigned to the experimental group were extensively trained on the use of the inControl Advice app and were asked to use it multiple times per day throughout the study. The smart insulin pens were able to communicate with the app and record the amount of insulin and timing of insulin administration. Participants in the control group did not have access to inControl Advice and were asked to use CGM and inject insulin with the study pens based on their normal dosing regimens.
HbA1c was collected at screening to assess whether the inclusion/exclusion criteria were met; at randomization and at the end of the study, HbA1c was collected again and analyzed in a central laboratory. The HbA1c collected at randomization was used as the baseline value. Baseline patient-reported outcomes (PRO) were collected at randomization, and final questionnaires were administered at the end of the study. Four validated questionnaires were administered to all participants: 1) the Hypoglycemia Fear Survey (HFS-II) (21), a 33-item survey that assesses fear of hypoglycemia; 2) the Hyperglycemia Avoidance Scale (22), a 21-item survey that reliably quantifies emotional and behavioral aspects of hyperglycemia avoidance; 3) the Diabetes Distress Scale (23), a 17-item scale that captures diabetes-related distress as well as four main domains of diabetes distress; and 4) Clarke’s Hypoglycemia Awareness scale (24), a scale that comprises eight questions characterizing a participant’s exposure to episodes of moderate and severe hypoglycemia as well as symptomatic responses to hypoglycemia. Two nonvalidated surveys were administered only to participants randomly assigned to the experimental group: the Technology Expectations Survey and the Technology Experience Survey. These questionnaires were adapted for this study from versions used in AID research (25) and are rated on a 5-point Likert scale. Items in the two questionnaires are identical, except future tense is used in the Technology Expectations Survey (administered at randomization), while past tense is used in the Technology Experience Survey (administered at the end of the study). These surveys yield two subscale scores (burdens and benefits), with higher scores indicating greater expected and perceived burdens or benefits related to technology.
Data Analysis
CGM data were used to compute several glycemic outcomes, including TIR (percentage of time spent with CGM values between 70 and 180 mg/dL) (26), TBR (percentage of time spent with CGM values <70 mg/dL), percentage of time spent with CGM values <60 mg/dL, percentage of time spent with CGM values <50 mg/dL, TAR (percentage of time spent with CGM values >180 mg/dL), time spent with CGM values >250 mg/dL, time spent with CGM values >350 mg/dL, low blood glucose index (LBGI) (27), high blood glucose index (HBGI) (28), and average daily risk range (ADRR) (29).
PRO were deidentified and scored following surveys’ instructions.
User Type Determination
Our per-protocol analysis aimed at understanding the impact of the system if it was sufficiently used. For this purpose, we defined a user score based on the average daily number of interactions with key functionalities: meal bolus calculator, bedtime advice, and exercise advice. Each user could gather up to 3 points in each category (for a total of 9), assigned as follows: 1) more than three meal announcement doses per day, 3 points; more than two, 2 points; and more than one, 1 point; 2) more than one bedtime advice request every 2 days, 3 points; more than one every 5 days, 2 points; and more than one, 1 point; and 3) more than one exercise advice request every 2 days, 3 points; more than one every 5 days, 2 points; and more than one every 10 days, 1 point.
Users scoring more than the center of the scale (4.5) were assigned to the active group; users below that threshold were considered nonactive. This classification of active and nonactive users was based on actual usage (with a 4.5 threshold equal to half of the total points) and not on relative terms (i.e., 50% top users), because this study aimed at evaluating the impact of actual usage on glycemic outcomes. Consequently, the active and nonactive groups turned out to be of unequal size.
Statistics
Statistical analysis was performed following an intent-to-treat (experimental vs. control) and per-protocol (active users, controls, and nonactive users) approach. The latter was defined after the end of data collection, not a priori.
The Kolmogorov-Smirnov test was used prior to data analysis to evaluate the normality of outcomes; outcomes that could not be distinguished from normal distribution were analyzed using paired and independent samples t tests; other outcomes were analyzed using Mann-Whitney U and Wilcoxon signed rank tests. Repeated measures ANOVA was used to assess change in time by group for glycemic and PRO variables, regardless of their distribution. Sample size was determined based on a prior study (17) and conservatively adjusted down to 0.16 in an effort to account for the introduction of CGM; based on a within-between repeated measures ANOVA of the primary outcome with 0.95 power and 0.05 significance, we determined an original sample size of 132, assuming 15% attrition with two unequal groups (2:1 randomization) and two repetitions. All statistical data analyses were performed using IBM SPSS Statistics v26.
Results
A total of 111 individuals with T1D on MDI treatment were recruited: 55 at the University of Virginia, 25 at Stanford University, and 31 at the Icahn School of Medicine at Mount Sinai. Across all sites, 12 did not meet the inclusion criteria, and 19 dropped out or withdrew from the trial. The final sample included 80 participants: 23 participants were randomly assigned to the control group (MDI + CGM) and 57 to the experimental group (DSS + MDI + CGM). The latter was split into two groups for data analysis purposes: 20 participants were identified as active users (described above; maximum reported score was 7), while 37 were classified as nonactive users (23 had scores ≤2, and 14 had scores between 2 and 4). At 80 completers, the a posteriori statistical power was reduced to ∼85% (we did not reach our target enrollment and experienced larger-than-expected attrition and therefore did not achieve the originally intended statistical power). Patient characteristics are listed in Table 1.
Table 1.
Demographic characteristics of study participants (intent-to-treat and per-protocol analyses)
| Variable | Experimental group | Control group | Active users | Nonactive users |
|---|---|---|---|---|
| Participants, n | 57 | 23 | 18 | 39 |
| Female sex, % | 59.6 | 47.8 | 77.8 | 51.3 |
| Age, years | 33.44 (14.15) | 39.91 (16.06) | 37.2 (13.49) | 31.69 (14.27) |
| Baseline HbA1c, % | 7.41 (1.18) | 7.68 (1.29) | 7.11 (1.61) | 7.55 (0.90) |
| Time since T1D diagnosis, years | 15.85 (12.58) | 15.26 (13.00) | 14.32 (11.83) | 16.56 (13.00) |
| Caucasian, % | 77 | 91 | 66.6 | 82.0 |
| BMI, kg/m2 | 27.42 (5.77) | 26.91 (6.85) | 29.34 (7.39) | 26.53 (4.71) |
| TDI, units/kg | 0.71 (0.26) | 0.72 (0.30) | 0.66 (0.24) | 0.73 (0.27) |
| CGM naïve, % | 47 | 52 | 55.6 | 43.6 |
| Current CGM users, % | 38.6 | 30.4 | 38.9 | 38.5 |
| Basal insulin, % of total daily insulin | 48.7 | 47.3 | 50.9 | 47.6 |
| Participants splitting basal insulin in two injections, % | 22.9 | 34.8 | 22.2 | 23.1 |
Data are presented as mean (SD) unless otherwise indicated.
TDI, total daily insulin.
The experimental and control groups were similar at randomization; however, significant differences at baseline were detected between active users and nonactive users. At baseline, active users had greater TIR (+11.0%; P = 0.034), lower TAR (−14.4%; P = 0.011), and lower mean CGM value (−27 mg/dL; P = 0.013) and HBGI (−5.1; P = 0.014) compared with nonactive users. Hyperglycemia Avoidance Scale scores showed that active users engaged more often in behaviors aimed at avoiding hypo- and hyperglycemia (P = 0.006) and tended to prefer lower blood glucose values compared with nonactive users (P = 0.011). Active users had higher expectations regarding the use of inControl Advice (lower expected burdens; P = 0.031 and higher expected benefits; P = 0.009) and were more likely to have hypoglycemia unawareness (P = 0.002). Details on the variables presented regarding differences between active and nonactive users at baseline are illustrated in Table 2.
Table 2.
Differences at baseline between active users and nonactive users (per-protocol analysis)
| Baseline variable | Active users | Nonactive users | P |
|---|---|---|---|
| Mean CGM value, mg/dL | 157.2 ± 46.2 | 184.8 ± 39.4 | 0.013 |
| HbA1c, % | 7.1 ± 1.5 | 7.6 ± 1.9 | 0.117 |
| TBR <70 mg/dL, % | 5.5 (1.1–8) | 3 (1.2–5.3) | 0.134 |
| TIR 70–180 mg/dL, % | 60.6 ± 21.1 | 49.6 ± 18.4 | 0.034 |
| TAR >180 mg/dL, % | 31.7 ± 19.2 | 46.1 ± 19.7 | 0.011 |
| Coefficient of variation, % | 35.4 ± 10.7 | 33 ± 5.6 | 0.181 |
| LBGI | 3.3 ± 2.8 | 1.9 ± 1.6 | 0.052 |
| HBGI | 9.8 ± 5.8 | 14.9 ± 7.9 | 0.014 |
| ADRR | 46.8 ± 14.3 | 48.2 ± 9.9 | 0.652 |
| HFS-II total | 41.3 ± 20.4 | 36.3 ± 17.6 | 0.352 |
| DDS total | 2.0 ± 0.8 | 2.2 ± 0.9 | 0.407 |
| HAS total | 43.8 ± 9.5 | 37.7 ± 8.0 | 0.017 |
| HAS: low BG preference | 8.8 ± 3.0 | 6.7 ± 2.7 | 0.011 |
| HAS: avoid extremes | 5.8 ± 2.2 | 4.0 ± 2.1 | 0.006 |
| Expected benefits | 71.4 ± 12.4 | 56.9 ± 21.7 | 0.009 |
| Expected burdens | 28.9 ± 15.9 | 39.2 ± 16.5 | 0.031 |
| Hypoglycemia awareness, R count | 2.4 ± 1.7 | 1.0 ± 0.9 | 0.002 |
Data are presented as mean ± SD or median (quartile range). Bold font indicates statistical significance.
BG, blood glucose; DDS, Diabetes Distress Scale; HAS, Hyperglycemia Avoidance Scale.
No significant differences were detected in glycemic or PRO outcomes at the end of the study between groups in the intent-to-treat analysis (control vs. experimental). Within groups, there were changes from baseline; participants in the control group had reductions in HbA1c (−0.4%; P = 0.009), coefficient of variation (−2.1%; P = 0.015), and ADRR (−3.5; P = 0.040), whereas participants in the experimental group had reductions in HbA1c (−0.3%; P = 0.001), higher TIR (+4.4%; P = 0.003), shorter time spent with CGM values <60 (−0.7%; P = 0.049) and >250 mg/dL (−3.3%; P = 0.010), lower HBGI (−1.3; P = 0.046), and lower ADRR (−2.4; P = 0.007). The PRO intent-to-treat analysis did not yield any significant differences between or within groups, except a significant decrease between expected and experienced benefits in the experimental group (P = 0.004).
In the per-protocol analysis, active users decreased their TBR significantly more than nonactive users (−2.6 vs. −0.6%; P = 0.019), along with their LBGI (−0.9 vs. 0; P = 0.008). When compared with their baseline, active users decreased their time spent with CGM values <60 (−1.7%; P = 0.049) and >250 mg/dL (−3.2%; P = 0.020), had lower LBGI (−0.9; P = 0.033), and had lower ADRR (−4.5; P = 0.040). They also showed increased TIR, which did not reach statistical significance (+5.1%; P = 0.055). On the other hand, nonactive users had significantly higher TIR (+3.8%; P = 0.023) and decreased their time spent at >350 mg/dL (−2.5%; P = 0.035) compared with their baseline data. The PRO per-protocol analysis identified significant changes in HFS-II scores between active and nonactive users throughout the study; the total score decreased significantly more in active users (P = 0.043), as did the worry subscale score (P = 0.016). Within groups, pre-post analysis identified significant decreases in HFS-II total score (P = 0.024) and HFS-II worry subscale score (P = 0.017) in active users. The abovementioned decrease between expected and experienced benefits occurred in active users only (P = 0.030).
All glycemic outcomes are presented in Table 3, while PRO are available in the Supplementary Material.
Table 3.
Glycemic outcome summary
| Control (n = 23) | DSS (n = 57) | P * | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Study | P † | Baseline | Study | P † | ||
| Intent-to-treat analysis | |||||||
| TIR 70–180 mg/dL, % | 54.9 ± 18.9 | 58.2 ± 18.8 | 0.202 | 53.3 ± 19.6 | 57.7 ± 16.3 | 0.003 | 0.855 |
| CGM | 177.2 ± 36.3 | 172.9 ± 39.8 | 0.605 | 175.4 ± 44.9 | 170 ± 33.4 | 0.129 | 0.988 |
| HbA1c, % | 7.7 ± 1.3 | 7.3 ± 1.3 | 0.009 | 7.4 ± 1.8 | 7.1 ± 0.9 | 0.001 | 0.844 |
| Time spent <50 mg/dL, % | 0.5 (0.1–1.2) | 0.2 (0.1–0.7) | 0.101 | 0.6 (0.2–1.5) | 0.4 (0.1–1.1) | 0.048 | 0.708 |
| Time spent <54 mg/dL, %‡ | 0.7 (0.3–1.8) | 0.3 (0.2–1.0) | 0.052 | 0.8 (0.2–2.4) | 0.6 (0.2–1.7) | 0.052 | 0.708 |
| Time spent <60 mg/dL, % | 1.5 (0.7–3) | 0.6 (0.3–1.9) | 0.068 | 1.7 (0.5–3.9) | 1 (0.4–2.7) | 0.049 | 0.938 |
| TBR (<70 mg/dL), % | 3.3 (1.5–6.4) | 2.1 (1–4.2) | 0.059 | 3.7 (1.2–6.8) | 2.6 (1–5.3) | 0.058 | 0.832 |
| TAR (>180 mg/dL), % | 40.9 ± 18.5 | 38.7 ± 20 | 0.396 | 41.2 ± 20.8 | 38.1 ± 17.8 | 0.050 | 0.916 |
| Time spent >250 mg/dL, % | 15 (6.3–24.1) | 14.4 (3.2–20.5) | 0.144 | 13.3 (4.6–23.7) | 9.7 (5.2–18.4) | 0.010 | 0.784 |
| Time spent >300 mg/dL, %‡ | 4.8 (2.1–7.9) | 4.5 (0.5–8.4) | 0.200 | 4.7 (0.9–9.9) | 2.9 (1.1–7.2) | 0.002 | 0.784 |
| Coefficient of variation, % | 33.5 ± 6.3 | 31.4 ± 5.2 | 0.015 | 33.8 ± 7.7 | 32.8 ± 4.8 | 0.166 | 0.248 |
| LBGI | 2 ± 1.3 | 1.7 ± 1.3 | 0.224 | 2.4 ± 2.2 | 2.1 ± 1.7 | 0.147 | 0.955 |
| HBGI | 13.4 ± 7.5 | 12.4 ± 7.9 | 0.330 | 13.2 ± 7.7 | 11.9 ± 6.6 | 0.046 | 0.961 |
| ADRR | 47.1 ± 13.1 | 43.6 ± 11.9 | 0.040 | 47.7 ± 11.5 | 45.3 ± 9.3 | 0.007 | 0.534 |
| Nonactive (n = 37) | Active (n = 20) | ||||||
| Per-protocol analysis | |||||||
| TIR 70–180 mg/dL, % | 49.6 ± 18.4 | 53.4 ± 16.5 | 0.023 | 60.6 ± 21.1 | 65.7 ± 12.8 | 0.055 | 0.988 |
| CGM | 184.8 ± 39.4 | 178.4 ± 34.7 | 0.118 | 157.2 ± 46.2 | 154.6 ± 24.9 | 0.629 | 0.271 |
| HbA1c, % | 7.6 ± 1.9 | 7.4 ± 0.9 | 0.007 | 7.1 ± 1.5 | 6.5 ± 0.8 | 0.025 | 0.068 |
| Time spent <50 mg/dL, % | 0.5 (0.2–1.1) | 0.4 (0.1–1) | 0.451 | 1.2 (0.1–3.3) | 0.4 (0.1–1.1) | 0.024 | 0.028 |
| Time spent <54 mg/dL, %‡ | 0.7 (0.3–1.6) | 0.6 (0.2–1.5) | 0.388 | 1.7 (0.2–4.2) | 0.6 (0.2–1.7) | 0.064 | 0.028 |
| Time spent <60 mg/dL, % | 1.2 (0.6–2.7) | 0.9 (0.4–2.4) | 0.414 | 2.9 (0.2–5.2) | 1.2 (0.4–3) | 0.049 | 0.026 |
| TBR (<70 mg/dL), % | 3 (1.2–5.3) | 2.4 (0.9–4.7) | 0.414 | 5.5 (1.1–8) | 2.9 (1.3–6.1) | 0.059 | 0.019 |
| TAR (>180 mg/dL), % | 46.1 ± 19.7 | 42.8 ± 17.8 | 0.059 | 31.7 ± 19.2 | 29.4 ± 14.5 | 0.568 | 0.378 |
| Time spent >250 mg/dL, % | 17.7 (6.7–27.9) | 14.5 (6.7–19.9) | 0.108 | 9.1 (1.7–18.4) | 6.8 (2–11.8) | 0.020 | 0.872 |
| Time spent >300 mg/dL, %‡ | 7.2 (1.3–13.2) | 4.7 (1.8–8.4) | 0.035 | 3.2 (0.1–7.5) | 1.9 (0.4–3.7) | 0.016 | 0.622 |
| Coefficient of variation, % | 33 ± 5.6 | 32.9 ± 5 | 0.918 | 35.4 ± 10.7 | 32.5 ± 4.4 | 0.083 | 0.069 |
| LBGI | 1.9 ± 1.6 | 1.9 ± 1.5 | 0.886 | 3.3 ± 2.8 | 2.4 ± 1.9 | 0.033 | 0.008 |
| HBGI | 14.9 ± 7.9 | 13.6 ± 7 | 0.090 | 9.8 ± 5.8 | 8.8 ± 4.4 | 0.295 | 0.464 |
| ADRR | 48.2 ± 9.9 | 46.8 ± 9.5 | 0.082 | 46.8 ± 14.3 | 42.3 ± 8.3 | 0.040 | 0.109 |
Data are presented as mean ± SD or median (quartile range) (for variables not normally distributed). Bold font indicates statistical significance.
Repeated measures ANOVA time x study arm P value.
Within-participant P value.
Added to the analysis during the peer review process.
Conclusions
For individuals with diabetes seeking support for glucose management, the inControl DSS can be effective in improving glycemic and psychobehavioral outcomes. Our intent-to-treat analysis was unable to identify statistically significant differences between the experimental and control groups; participants in both groups showed improved glycemic outcomes during the trial in both HbA1c and glycemic variability (ADRR). Nonetheless, within-group comparisons showed that TIR; time spent <60, >180, or >250 mg/dL, and HBGI were significantly lower than baseline values for the DSS group, while we could not confirm these changes in the CGM group; conversely, the coefficient of variation seemed to be statistically improved in the CGM group only. Review of data from participants who actively engaged with the DSS used in this trial, through the per-protocol analysis, supported that some of these improvements were caused by the use of InControl. When compared with nonactive users, active participants spent a significantly shorter time in hypoglycemia and had a lower risk of hypoglycemia (LBGI). This suggests that the actual use of the system, and not just access to it, is key to achieving better glycemic results. Hypoglycemia reduction was not only identified by researchers but also noted by active users; in fact, they reported a significant reduction in their hypoglycemia-related emotional distress and anxiety. The creation of the user score led to the identification of predominant characteristics at baseline in individuals who ended up using the system consistently. At baseline, active users already had better glycemic control, with higher TIR and lower TAR and HBGI, than nonactive users; however, they tended to keep their blood glucose lower and experienced more hypoglycemic events, which led to greater concerns related to low blood glucose as well as higher prevalence of hypoglycemia unawareness. Because of a lack of data on participants randomly assigned to the control group regarding insulin dosage, we are unable to make statements about insulin changes.
The optimal form of a DSS and how it engages the user remain to be determined. The fairly low engagement threshold used to separate active versus nonactive users led to only 35% of users being classified as active, even though typical enrollees in diabetes-related technology trials are historically more willing to use technology in the management of their diabetes than the population at large. Understanding the factors leading to active use is undoubtedly a key element to a future successful DSS. Even though the protocol did not include follow-up interviews or focus groups to collect qualitative data about the users’ experience with the InControl app, clinical research coordinators reported that many participants complained about connectivity issues, alarm fatigue, and the need for high-quality data for the system to function properly. The current system was relying on strict adherence to intensive insulin therapy, with announced meals and corresponding insulin doses computed through a bolus calculator; this design may create additional hurdles in the adoption of this technology in larger populations. Participants who volunteer for clinical trials are generally already engaged in their diabetes management and are motivated to participate. More than 800 potentially interested individuals with T1D on MDI were contacted; however, most of the respondents did not pass the prescreening, were not interested in becoming part of a trial testing this kind of system, or were interested but not available for early-stage studies like ours; therefore, they were never formally enrolled. This likely led to underenrollment (N = 111 vs. 132) and higher-than-anticipated dropout rates (∼25 vs. 15%), limiting our statistical power. All of our participants, regardless of the study arm to which they were randomly assigned, benefitted from the uninterrupted use of state-of-the-art CGM and were provided longer-acting, more stable basal insulin. These interventions, along with the study effect, had a positive impact on the outcomes of participants in the control group as well as participants who had suboptimal engagement with the study device (e.g., improved HbA1c by 0.3–0.4%). Reproducibility in a more diverse MDI population, challenges with adherence to protocol, and the confounding effect of CGM use on glycemic outcomes and PRO remain the main limitations of this study.
In this study, the randomized introduction of the DSS was associated with a major therapy change related to insulin treatment and the adoption of a CGM device in both experimental and control groups, leading to improvements in therapeutic outcomes in both groups, which potentially confounded the assessment of the specific benefit of the DSS. Moreover, active DSS users can be patients who are already very much engaged in their self-management, and it could be argued that they would have improved their glucose control and PRO even without DSS use.
The use of real-time CGM in association with an insulin pen with memory led to the creation of a broad, detailed, and reliable data set collected from a population of patients on MDI who are usually self-reporting their bolus timing and dosage; for example, it allowed for a subanalysis of 41,173 meals in 24 participants. From this analysis, 13% of the meal boluses were late, and 14% were missed (30); these caused an additional burden on the DSS when recommending dosage changes.
Nonetheless, as shown by our active versus nonactive analysis, use of the DSS presented in this trial (InControl app) seems to be a feasible option for people on MDI who are already in relatively good control but want to reach better outcomes and do not wish to or cannot transition to CSII. People using MDI can seamlessly transition to smart insulin pens, which, in conjunction with CGM, make for an extraordinary depth of information that a well-designed DSS should leverage for improving quality of care. As with other DSS (PEPPER (18) and ABC4D (19)), which have been proven to be safe but have had marginal efficacy for users, further evaluation of factors important to individuals with T1D is needed to broaden interest among those with less optimal glycemic control in the use of these systems.
Article Information
Acknowledgments. The authors acknowledge Chad Rogers, Stephen Patek, Colin Steele, Patrick Keith-Hynes, Antoine Robert, Benton Mize, and Laurie Wright from TypeZero Technologies for their provision of the DSS, unwavering technical support, and commitment to see this study through.
Funding. This work was supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01DK051562 and DP3DK101055), and material support was provided by Dexcom, Novo Nordisk, and TypeZero Technologies.
Duality of Interest. S.A. receives research support from Medtronic (paid to institution). G.O., S.O., and C.Levi. receive research support from Tandem Diabetes Care, Insulet, Dexcom, and Abbott Laboratories (paid to institution). L.E. has received consulting fees from Tandem Diabetes Care and Ypsomed. D.W.L. receives research support from Tandem Diabetes Care and Insulet (paid to institution). C. Levy receives research support from Tandem Diabetes Care, Insulet, Dexcom, and Abbott Laboratories (paid to institution) and has served as a consultant for Dexcom and Eli Lilly. B.B. has received research support from Tandem Diabetes Care, Insulet, Dexcom, JDRF, the National Institutes of Health, and the Helmsely Foundation (paid to institution) and served as a consultant for Novo Nordisk, Medtronic Diabetes, ConvaTec, Dexcom, Insulet, and Tandem Diabetes Care. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.B. participated in data analysis and manuscript writing and editing. S.A. was the principal investigator (PI) at the University of Virginia (UVA) and participated in study design, contributed to manuscript writing and revisions, led participant enrollment, and monitored site safety and assumes responsibility and accountability for UVA site data. L.N., G.O., and J.R. participated in study enrollment, conducted study visits, and contributed to manuscript revisions. S.O. participated in study enrollment, conducted study visits, performed study device and data management, and contributed to manuscript edits. L.H., C.Levi., and L.E. participated in study enrollment, conducted study visits, and contributed to manuscript revisions. D.W.L. participated in study enrollment and contributed to manuscript revisions. C. Levy was the PI at the Icahn School of Medicine and contributed to manuscript writing and revisions, led participant enrollment, and monitored site safety and assumes responsibility and accountability for Icahn School of Medicine site data. B.B. was the PI at Stanford and contributed to manuscript writing and revisions, led participant enrollment, and monitored site safety and assumes responsibility and accountability for Stanford site data. M.D.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT03093636, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.16891267.
References
- 1. American Diabetes Association . Standards of medical care in diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S1–S183 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Accessed 16 November 2021. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 3. Blackman SM, Raghinaru D, Adi S, et al. Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes 2014;15:564–572 [DOI] [PubMed] [Google Scholar]
- 4. Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 5. Lansang MC, Modic MB, Sauvey R, et al. Approach to the adult hospitalized patient on an insulin pump. J Hosp Med 2013;8:721–727 [DOI] [PubMed] [Google Scholar]
- 6. Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting, and research needs: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015;38:716–722 [DOI] [PubMed] [Google Scholar]
- 7. Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care 2018;41:1579–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanenbaum ML, Adams RN, Hanes SJ, et al. Optimal use of diabetes devices: clinician perspectives on barriers and adherence to device use. J Diabetes Sci Technol 2017;11:484–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007;30:549–554 [DOI] [PubMed] [Google Scholar]
- 10. Beck RW, Riddlesworth T, Ruedy K, et al.; DIAMOND Study Group . Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 11. Contreras I, Vehi J. Artificial intelligence for diabetes management and decision support: literature review. J Med Internet Res 2018;20:e10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tyler NS, Mosquera-Lopez CM, Wilson LM, et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat Metab 2020;2:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forlenza GP. Use of artificial intelligence to improve diabetes outcomes in patients using multiple daily injections therapy. Diabetes Technol Ther 2019;21(Suppl. 2):S24–S28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Connor PJ, Sperl-Hillen JM, Fazio CJ, Averbeck BM, Rank BH, Margolis KL. Outpatient diabetes clinical decision support: current status and future directions. Diabet Med 2016;33:734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia P, Zhao P, Chen J, Zhang M. Evaluation of clinical decision support systems for diabetes care: an overview of current evidence. J Eval Clin Pract 2019;25:66–77 [DOI] [PubMed] [Google Scholar]
- 16. Roshanov PS, Fernandes N, Wilczynski JM, et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013;346:f657. [DOI] [PubMed] [Google Scholar]
- 17. Breton MD, Patek SD, Lv D, et al. Continuous glucose monitoring and insulin informed advisory system with automated titration and dosing of insulin reduces glucose variability in type 1 diabetes mellitus. Diabetes Technol Ther 2018;20:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avari P, Leal Y, Herrero P, et al. Safety and feasibility of the PEPPER adaptive bolus advisor and safety system: a randomized control study. Diabetes Technol Ther 2021;23:175–186 [DOI] [PubMed] [Google Scholar]
- 19. Reddy M, Pesl P, Xenou M, et al. Clinical safety and feasibility of the advanced bolus calculator for type 1 diabetes based on case-based reasoning: a 6-week nonrandomized single-arm pilot study. Diabetes Technol Ther 2016;18:487–493 [DOI] [PubMed] [Google Scholar]
- 20. Garg AX, Adhikari NKJ, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–1238 [DOI] [PubMed] [Google Scholar]
- 21. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care 2011;34:801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh H, Gonder-Frederick L, Schmidt K, et al. Assessing hyperglycemia avoidance in people with type 1 diabetes. Diabetes Manag (Lond) 2014;4:263–271 [Google Scholar]
- 23. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 24. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522 [DOI] [PubMed] [Google Scholar]
- 25. Weissberg-Benchell J, Hessler D, Polonsky WH, Fisher L. Psychosocial impact of the bionic pancreas during summer camp. J Diabetes Sci Technol 2016;10:840–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 28. Kovatchev B, Cox DJ, Gonder-Frederick L, Clarke WL. Methods for quantifying self-monitoring blood glucose profiles exemplified by an examination of blood glucose patterns in patients with type 1 and type 2 diabetes. Diabetes Technol Ther 2002;4:295–303 [DOI] [PubMed] [Google Scholar]
- 29. Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006;29:2433–2438 [DOI] [PubMed] [Google Scholar]
- 30. Norlander LM, Anderson S, Levy CJ, et al. Late and missed meal boluses with multiple daily insulin injections. Diabetes 2018;67(Suppl. 1):A259 [Google Scholar]