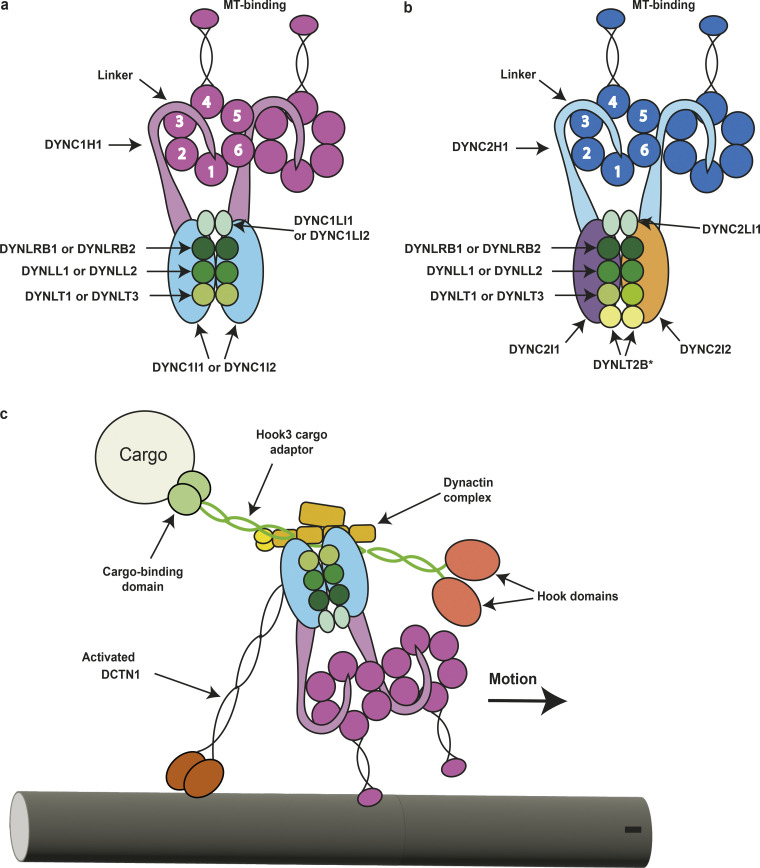
Figure 1.
Cytoplasmic dynein complexes. (a) The cytoplasmic dynein 1 complex. The DYNC1H1 protein heavy chains have large globular heads at the C-termini that are composed of a ring of six AAA+ domains. The microtubule-binding domains are located at the tips of antiparallel coiled coils that derive from AAA4. The linker/N-terminal domains connect the AAA rings and the intermediate and light chains. (b) The cytoplasmic dynein 2 complex. The DYNC2H1 protein heavy chains power retrograde IFT and have the same general domain organization as DYNC1H1. However, the tails of the two heavy chains fold differently due to an asymmetry imposed by the two different intermediate chains: one is straight while the other forms a zigzag shape and interacts with the IFT-B train (Toropova et al., 2019). The linker/N-terminal domain connects the AAA ring and the intermediate and light chains. *It remains unknown whether the DYNLT2B protein forms a homodimer or a heterodimer with another Tctex-type light chain. (c) Schematic showing the interaction between the dynein 1 and dynactin complexes. The adapter molecule affects the type of cargo bound; in this figure, the hook microtubule tethering protein 3 (HOOK3)–encoded protein is acting as a cargo adapter.