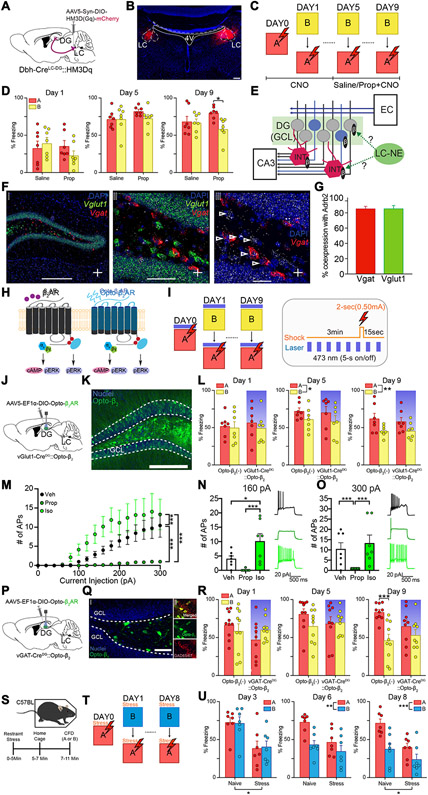
Fig. 4. Pharmacological and optogenetic modulation of noradrenergic signaling in DG interneurons and GCs during contextual fear discrimination.
(A) Schematic of experimental approach depicts infection of LC-NE cells using Dbh-Cre mouse line with DREADD system activating HM3Dq signaling chemogenetically. (B) Representative image depicting expression of HM3Dq in LC-NE cells of Dbh-Cre mice. Scale bar = 50 μm. (C) CFD task with CNO injection 30 minutes prior to task. Starting on day 5, animals received injection of either saline (Dbh-Cre::Saline) or propranolol (Dbh-Cre::Prop) 30 minutes prior to CNO injection. (D) Chemogenetic activation of the LC-NE system impairs behavioral pattern separation in mice receiving saline prior to CNO (n = 7), but not in those that received propranolol prior to CNO (n = 7). Animals receiving propranolol prior to CNO were able to successfully discriminate on the ninth day of training, but animals receiving saline prior to CNO were not (Saline: Day 1A: 33.3 ± 8.94; Day 1B: 39.8 ± 6.93; Day 5A: 71.4 ± 4.46; Day 5B: 71.8 ± 6.74; Day 9A: 68.3 ± 6.98; 9B: 66.9 ± 4.79; Propranolol: Day 1A: 35.8 ± 6.90; Day 1B: 22.8 ± 6.31; Day 5A: 82.5 ± 2.24; Day 5B: 73.3 ± 4.83; Day 9A: 80.0 ± 3.19; Day 9B: 59.1 ± 5.50; Two-way ANOVA for Saline vs Propranolol, A vs B: Day 1: Group: F(1,12) = 0.665, p = 0.431; Context: F(1,12) = 0.361, p = 0.559; Group × Context: F(1,12) = 3.29, p = 0.0947; Day 5: Group: F(1,12) = 1.31, p = 0.274; Context: F(1,12) = 1.15, p = 0.305; Group × Context: F(1,12) = 1.39, p = 0.261; Day 9: Group: F(1,12) = 0.109, p = 0.747; Context: F(1,12) = 6.34, p = 0.0271; Group × Context: F(1,12) = 4.78, p = 0.0494). (E) Diagram depicts hypothesis regarding potential LC-DG circuitry. Neurons in the Entorhinal Cortex (EC) send projections to the DG through the perforant pathway. The DG sends projections to pyramidal cells in the CA3 through mossy fibers. LC-NE projections may modulate granule cells (GC) directly during a new experience, or may modulate local interneurons (INT), causing strong tonic inhibition of GCs. (F) In-situ hybridization depicting coronal images of colocalization of Adrb2 (white), Vglut1 (green), and Vgat (red) within the DG of wild-type mice. (G) Quantification of Vgat and Vglut1 coexpression with Adrb2. (H) Diagram depicts intracellular pathway of Opto-β2 adrenergic receptors (ARs). (I) CFD task with optogenetic β2-AR modulation of DG GCs or interneurons (left). Pattern of laser stimulation and shock (only in context A) during CFD (right). (J) Schematic of experimental approach depicts infection of DG GCs using vGlut1-Cre mouse line with chimeric light-sensitive adrenergic receptors (vGlut1-CreDG::Opto-β2). (K) Opto-β2 ARs were expressed in the GCL. Scale Bar = 200 μm (L) Acute photoactivation of Opto-β2 ARs in DG GCs did not impair discrimination in mice expressing Opto-β2 in LC-DG projections or wild-type controls. Opto-β2(−) controls (n = 7) were able to discriminate on the ninth day of training, and vGlut1-CreDG::Opto-β2 mice (n = 7) were also able to discriminate on the ninth day of training (Opto-β2(−): Day 1A: 52.2 ± 5.64; Day 1B: 49.7 ± 7.97; Day 5A: 72.2 ± 4.20; Day 5B: 61.3 ± 5.82; Day 9A: 62.0 ± 7.09; Day 9B: 45.6 ± 2.68; vGlut1-CreDG::Opto-β2: Day 1A: 56.9 ± 8.37; Day 1B: 49.2 ± 7.55; Day 5A: 70.4 ± 7.88; Day 5B: 58.3 ± 7.87; Day 9A: 58.1 ± 5.58; Day 9B: 45.6 ± 4.07; Two-way ANOVA for vGlut1-CreDG::Opto-β2 vs Opto-β2 (−), A vs B: Day 1: Group: F(1, 12) = 0.0468, p = 0.832; Context: F(1,12) = 1.49, p = 0.246; Group × Context: F(1,12) = 0.398, p = 0.540; Day 5: Group: F(1, 12) = 0.0913, p = 0.768; Context: F(1,12) = 5.20, p = 0.0417; Group × Context: F(1,12) = 0.0151, p = 0.904; Day 9: Group: F(1, 12) = 0.101, p = 0.757; Context: F(1,12) = 16.14, p = 0.0017; Group × Context: F(1,12) = 0.310, p = 0.588). Both groups received optogenetic light during trial, shaded bar denotes successful stimulation of opsin. (M) Whole-cell patch clamp recordings of depolarization elicited action potential firing. 5μM Isoproterenol increased (p<0.0001) while 50μM Propranolol decreased (p<0.0001) the number of depolarization elicited action potentials (Depolarization Step: F(15,288) = 9.303, p<0.0001; Drug: F(2,288) = 59.84, p<0.0001; Depolarization Step x Drug: F(30,288) = 2.207, p = 0.0005). (N) Comparison of action potential firing between vehicle, isoproterenol, and propranolol at 160 pA depolarization step (Derived from Fig. 4I: Veh vs Iso: p=0.0385; Prop vs Iso: p=0.0005). (O) Comparison of action potential firing between vehicle, isoproterenol, and propranolol at 300 pA depolarization step (Derived from Fig. 4I: Veh vs Prop: p=0.0007; Veh vs Iso: p=0.2752; Prop vs Iso: p<0.0001). (P) Schematic of experimental approach depicts infection of DG interneurons using vGAT-Cre mouse line with chimeric light-sensitive adrenergic receptors (vGAT-CreDG::Opto-β2). (Q) Opto-β2 ARs were expressed in the hilus area (left) and co-expressed with GAD67 (right). Scale Bars = 50 μm (left), 15 μm (right). (R) Acute photoactivation of Opto-β2 ARs in DG interneurons impaired discrimination in mice expressing Opto-β2 in LC-DG projections but not in wild-type controls. Opto-β2(−) controls (n = 10) were able to discriminate on the ninth day of training, while vGAT-CreDG::Opto-β2 mice (n = 9) were not able to discriminate throughout the training (Opto-β2(−): Day 1A: 67.1 ± 5.63; Day 1B: 58.7 ± 9.36; Day 5A: 81.2 ± 6.50; Day 5B: 65.2 ± 7.25; Day 9A: 83.6 ± 3.41; Day 9B: 46.3 ± 7.96; vGAT-CreDG::Opto-β2: Day 1A: 47.3 ± 8.48; Day 1B: 60.5 ± 4.97; Day 5A: 68.5 ± 8.90; Day 5B: 70.1 ± 5.27; Day 9A: 65.1 ± 7.92; Day 9B: 53.4 ± 7.11; Two-way ANOVA for vGAT-CreDG::Opto-β2 vs Opto-β2(−), A vs B: Day 1: Group: F(1, 17) = 0.862, p = 0.366; Context: F(1,17) = 0.352, p = 0.561; Group × Context: F(1,17) = 7.167, p = 0.016; Day 5: Group: F(1, 17) = 0.196, p = 0.664; Context: F(1,17) = 2.175, p = 0.159; Group × Context: F(1,17) = 3.281, p = 0.088; Day 9: Group: F(1,17) = 0.407, p = 0.531; Context: F(1,17) = 44.286, p < 0.001; Group × Context: F(1,17) = 12.026, p = 0.003). Both groups received optogenetic light during trial, shaded bar denotes successful stimulation of opsin. (S) Depiction of experimental protocol. Wild-type mice either received restraint stress or were handled normally for approximately 5 minutes, then placed back in the home cage for another 5 minutes prior to contextual fear discrimination (CFD) training. (T) CFD task with restraint stress administration replacing optogenetic activation. (U) Analysis of progressive days of training show that naïve animals successfully began to discriminate context A from context B, while stress-treated animals were unable to successfully discriminate. Additionally, stress-treated animals showed significantly lower freezing levels than naïve animals in the early part of training. However, significant differences are seen at the end of training (Day 3: Group: F(1,9.76) = 6.137, p = 0.033; Day 6: Group: F(1,9.46) = 4.044, p = 0.073; Context: F(1,13.8) = 21.429, p < 0.001; Days × Context: F(1, 13.8) = 3.824, p = 0.071; Day 8: Group: F(1,10.39) = 7.109, p = 0.023; Context: F(1,14.87) = 24.6072, p < 0.001; Days × Context: F(1, 14.87) = 3.322, p = 0.085). All data are mean ± SEM. *p < 0.05, ***p < 0.005.