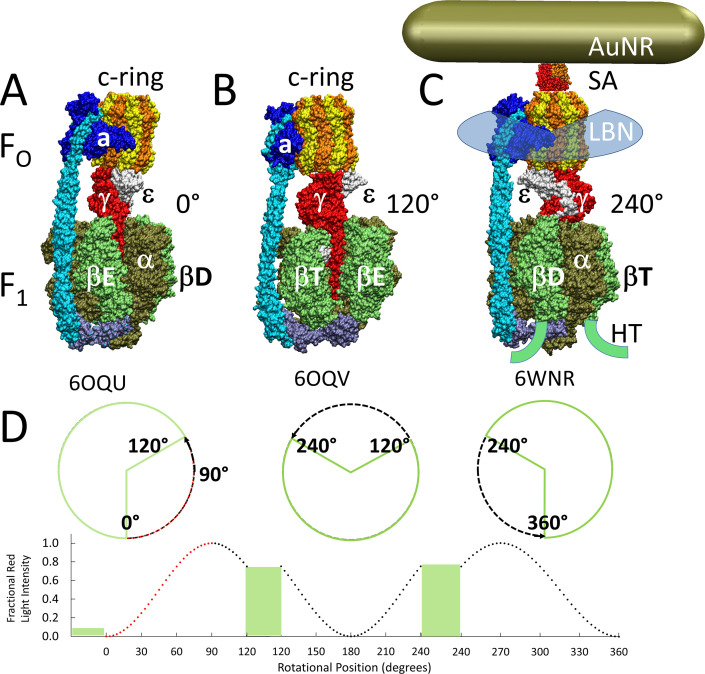
Figure 1. Cryo-EM structures of F1FO ATP synthase inhibited by ADP in three rotary states, and measurement of changes in rotational position between catalytic dwells.
(A) Rotational state-1, pdb-ID 6OQU (Sobti et al., 2020). (B) State-2, pdb-ID 6OQV, with rotor 120° counterclockwise (CCW) from (A) where subunit-α is not shown to reveal subunit-γ. (C) State-3, pdb-ID 6WNR, with rotor 240° CCW from (A) showing microscope slide assembly of F1FO embedded in a lipid bilayer nanodisc (LBN) for rotation measurements. His6-tags (HT) on β-subunit C-termini enabled attachment to slide, while the gold nanorod (AuNR) coated with streptavidin (SA) bound to the biotinylated subunit c-ring. (D) Rotational position of single F1FO molecules versus time was monitored by intensity changes of polarized red light scattered from the AuNR in the presence of 1 mM Mg2+ATP, which enabled F1-ATPase-dependent 120° CCW power strokes between catalytic dwells (green bars). Prior to data collection at 200 kHz, a polarizer in the scattered light path was rotated to minimize intensity during one of the three catalytic dwells. Light intensity increased to a maximum upon rotation by 90° during the subsequent CCW 120° power stroke. For each molecule the angular dependence of these power strokes versus time was analyzed.