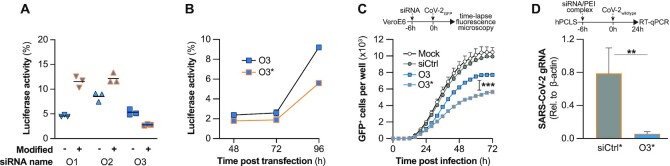
Figure 5.
Chemically modified siRNA inhibits SARS-CoV-2 replication ex vivo in the human lung. (A) ORF1-targeting siRNAs were chemically modified using a clinically validated chemistry (for details, see Material and Methods and Supplementary Table S4) and the activity compared to chemically non-modified versions of the siRNAs using luciferase reporters. For this, siRNAs and luciferase reporter plasmids were co-transfected into HEK293T cells and after 24 h luciferase activities determined. Values were normalized to a control group transfected with the respective luciferase reporter and the control siRNA with identical chemistry. (B) Effect of chemical modifications on the duration of RNAi-silencing by siRNA O3 was compared using the same experimental setup as in (A), and luciferase activity was determined at indicated time points. (C) Antiviral activity of the modified and non-modified version of siRNA O3 were compared using the rSARS-CoV-2-GFP model. siRNAs were transfected into VeroE6 at a concentration of 50nM. 6h later, cells were infected with rSARS-CoV-2-GFP (MOI1), and GFP+ cells were quantified using the Incucyte S3 system. (D) To validate the approach in a highly relevant model of the human lung, the chemically modified siRNA O3 was complexed with polyethylenimine (PEI), and transfected into human precision cut lung slices (hPCLS; 100nM), which were infected with wildtype SARS-CoV-2 (MOI 1) 6h later. RNA was extracted from hPCLS harvested 24h p.i. and viral replication quantified by RT-qPCR for SARS-CoV-2 gRNA (normalized to β-actin expression). Experiments shown in (A–C) were performed using three biological replicates, (D) using five replicates. Horizontal bars in (A, D) indicate mean, error bars in (B–D) S.E.M. n.s., non-significant; **P < 0.01; ***P < 0.001.