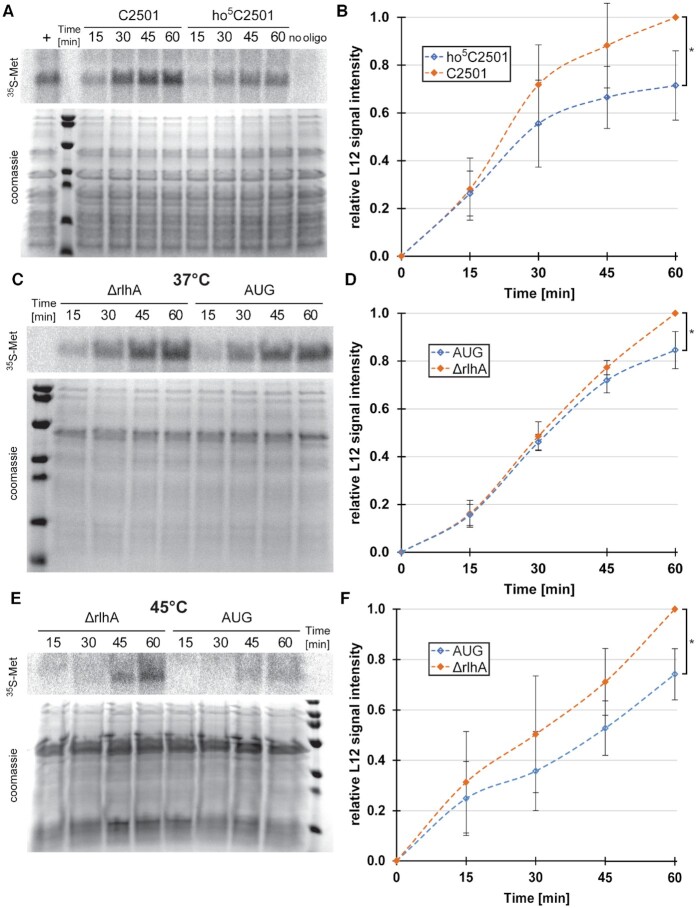
Figure 5.
ho5C2501 slows down translation in vitro. (A) Representative phoshporimager scan of in vitro translated 35S-labeled L12 protein (top) and coomassie stained proteins as loading control at the bottom. In vitro translations were performed with in vitro reconstituted 50S subunits utilizing an atomic mutagenesis approach with an unmodified (C2501) or modified (ho5C2501) RNA oligo. No oligo was added during 50S reconstitution as a negative control (no oligo). As a positive control, in vitro reconstitution was performed with in vitro transcribed canonical full-length 23S rRNA (+). (B) Quantification of average signal intensities at the marked time points with the signal intensity of C2501 (t = 60 min) set to 1. Error bars indicate standard deviation, significance was calculated using paired Student's t-test (P = 0.029, n = 4). (C) Same as in (A), but in vitro translation was performed at 37°C with in vivo-derived 50S subunits isolated from an unmodified knockout strain (ΔrlhA) and an almost quantitatively modified rlhA overexpression strain (AUG). (D) Quantification of average signal intensities at the marked time points with the signal intensity of ΔrlhA (t = 60 min) set to 1. Error bars indicate standard deviation, significance was calculated using paired Student's t-test (P = 0.042, n = 4). (E) Same as in (C), but in vitro translation was performed at 45°C. (F) Quantification of average signal intensities at the marked time points with the signal intensity of ΔrlhA (t = 60 min) set to 1. Error bars indicate standard deviation, significance was calculated using paired Student's t-test (P = 0.022, n = 4).