Abstract
Background
There is currently no clinically validated biomarker to predict respiratory compromise in sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Cycle threshold time (Ct), absolute lymphocyte count (AL) and neutrophil:lymphocyte ratio (NLR) have been previously evaluated for this purpose. We hypothesized that the combination of these parameters at presentation may be predictive of hypoxia (oxygen saturation <92%).
Methods
Data were collected on 118 patients with SARS-CoV-2 infection between May 2020 and April 2021. Demographics, clinical parameters and laboratory and radiological investigation results were recorded. Respiratory compromise (RC) was defined based on symptoms and signs, hypoxia and chest X-ray abnormalities.
Results
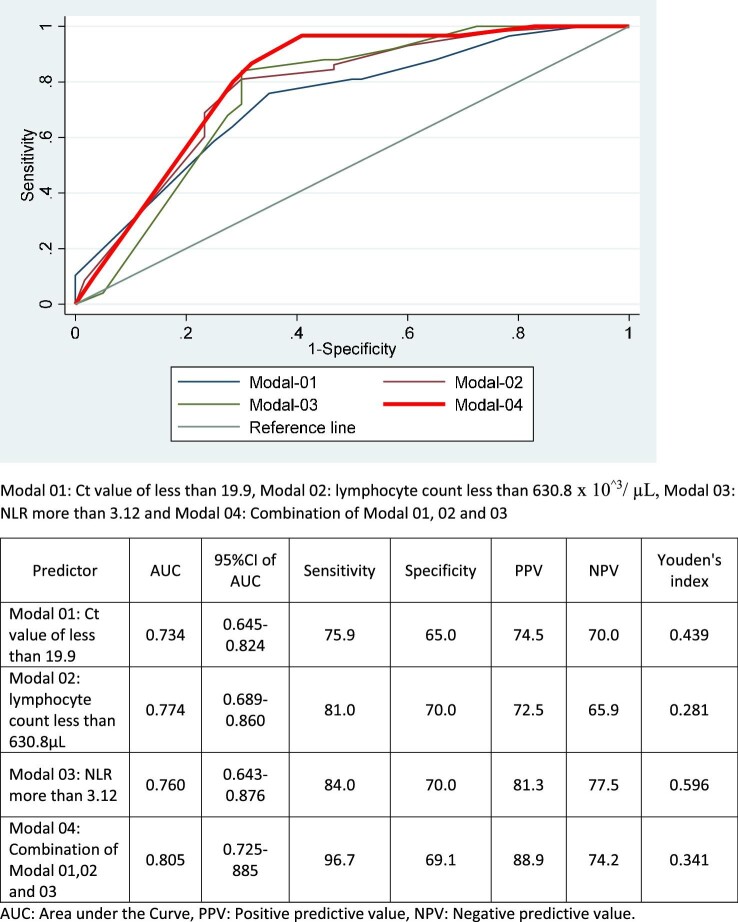
RC occurred in 61 (51.7%) of patients. The Ct, AL and NLR at median day 3 of illness were significantly different between patients with and without RC (Ct, RC vs not: 19.46±2.64 vs 22.62±3.37, p=0.0001; AL, RC vs not: 531.49±289.09 vs 764.69±481.79, p=0.0001; NLR, RC vs not: 3.42±0.75 vs 2.59±0.55, p=0.0001). Receiver operating characteristics analysis showed that a Ct <19.9, AL <630.8×103/μL and NLR >3.12 at median day 3 of symptoms was predictive of hypoxia on day 7 of illness (area under the curve 0.805, sensitivity 96.7%, specificity 69.1%). The predictive value for the parameters combined was significantly superior to their individual predictive power.
Conclusions
Ct, AL and NLR used in combination on day 3 of symptoms are predictive of hypoxia on day 7 of SARS-CoV-2 illness.
Keywords: absolute lymphocyte count, cycle threshold time, neutrophil:lymphocyte ratio, respiratory compromise, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that causes coronavirus disease 2019 (COVID-19) and was first identified in Wuhan, China in December 2019.1 While COVID-19 can have a variety of presentations, upper respiratory tract symptoms are the most common.2–4 Hypoxia secondary to COVID-19 infection is a result of an acute inflammatory response affecting the lungs.5,6 The incidence of COVID-19-related hypoxia ranges from 20 to 40% and is the main cause of mortality related to COVID 19.6–10 Early identification of COVID-19-related hypoxia is therefore essential.
Respiratory compromise (RC) in COVID-19 is defined in Sri Lanka based on national guidelines.11 Specific parameters include epidemiological history, clinical symptoms, vital signs (respiratory rate 20–30 breathes/min, heart rate 100–120 bpm, oxygen saturation on room air <92% by pulse oximeter) and chest X-ray (CXR) findings. Relevant CXR findings included ground glass opacities, peripheral consolidation and effusions.11
Granulocyte colony stimulating factor (G-CSF), interferon-inducible protein-10 (IP-10), monocyte chemoattractant protein-1 (MCP-1) and inflammatory macrophage protein-1α have been proposed as predictors of severe COVID-19.12 However, these parameters have not been validated for clinical use and are not readily available in developing countries. More easily accessible investigations that are proposed to predict the severity of COVID-19 include troponin, creatine kinase MB, N-terminal prohormone of brain natriuretic peptide, C-reactive protein (CRP) and lactate dehydrogenase.13,14 The predictive value of these biomarkers was demonstrated during the first week of hospital admission.15 The standard method for diagnosis of COVID-19 is via real-time reverse transcription polymerase chain reaction (RT-PCR).16 Real-time RT-PCR cycle threshold (Ct) values represent the number of amplification cycles required for the target gene to exceed a threshold level. Ct values are therefore inversely related to viral load17 and lower Ct values are associated with adverse outcomes in COVID-19.18
Increased lymphocyte apoptosis and T cell deregulation (attributed to high levels of interleukin-6 [IL-6]) as well as Fas–Fas ligand interactions may contribute to the immune deregulation leading to COVID-19-related RC.19,20 Reduced CD4+ and CD8+ T cell subsets were also shown to be predictors for severe COVID-19.10 A low absolute lymphocyte (AL) count on admission was associated with poor outcomes in a meta-analysis of patients with COVID-19 and was predictive for which patients may benefit from steroid therapy.21,22 Neutrophil activation combined with older age and an increase of neutrophil extracellular traps contribute to COVID-19-related RC.23–25 Also, neutrophilia as well as an elevated neutrophil:lymphocyte ratio (NLR) have been reported as predictive factors for adverse outcomes in COVID-19.25–28 We hypothesized that Ct, AL and NLR, when used collectively, may have a stronger predictive value for respiratory compromise than when these parameters are used individually.
Methods
Study design
A retrospective study was conducted on adult patients admitted to Nawaloka Hospital, Colombo, Sri Lanka with confirmed SARS-CoV-2 infection between May 2020 and April 2021.
Study population
We included 118 patients (>18 y of age) with SARS-CoV-2 infection confirmed by RT-PCR from nasopharyngeal swabs (AccuPower SARS-CoV-2 RT-PCR kit, Bioneer, Daejeon, South Korea).29 Positive test results were re-analysed with the RealStar SARS-CoV-2 RT-PCR Kit 1.0 (Altona Diagnostics, Hamburg, Germany)30 for confirmation. The reliability of the results was ensured through internal quality control measures as well as participation in external quality assurance schemes in collaboration with the Medical Research Institute of Sri Lanka. The onset of illness was determined based on guidelines from the Ministry of Health of Sri Lanka. Relevant symptoms included fever, cough, difficulty breathing and sore throat.31
Patients <18 y of age and those with immunosuppression were excluded from the study. Immunosuppression was defined as the presence of human immunodeficiency virus infection, solid organ or stem cell transplantation, neutropenia or ongoing immunosuppressive treatment.
Data collection
Demographic, clinical and laboratory data
Demographic and clinical parameters, including presenting symptoms, vital signs and laboratory and radiological investigations, were extracted by a trained study team member from the available medical records. Pulse oximetry was performed based on the manufacturer's guidelines (see Supplementary Data 1). CXR images were analysed by two experienced consultant radiologists who were blinded to the clinical data and final decisions were reached by consensus. For disagreement in interpretation between the two radiologists, a third radiologist adjudicated a final decision. AL and neutrophil count data were extracted from the XN-1000 automated full blood count analyser (Sysmex, Kobe, Japan).
Data analysis
Continuous variables were described using mean and standard deviation (SD) values. Differences between groups were compared using Student's t-test (parametric data) or the Mann–Whitney U-test (non-parametric data). Categorical data were expressed in total numbers and percentages and compared using a Z-test for two proportions and were compared using the χ2 test. For receiver operating characteristics (ROC) curve analysis, specific cut-off values for AL, Ct and NLR were used to determine the area under the curve (AUC) and sensitivity and specificity values. p-Values <0.05 were considered statistically significant. The average oxygen saturation level on day 7 of illness was used as the independent variable for the ROC analysis. Data were analysed using the SPSS version 16 (SPSS, Chicago, IL, USA) and Stata version 12 (StataCorp, College Station, TX, USA).
Results
We extracted records of 118 patients, 53.4% males, with a mean age of 50.22±15.11 y. Half of the patients 59/118 (50.0%) did not have any comorbidities and were admitted on day 3 of illness. The median length of hospital stay was 14 d. The most common presenting symptom was fever (84/118 [71.2%]) and intensive care unit (ICU) admission was required in 61/118 (51.7%) patients.
Patients were treated based on national guidelines11 and World Health Organization guidelines.31 Patients with RC comprised 51.7% of cases (61/118), all of whom were treated with oxygen, intravenous dexamethasone and prophylactic low molecular weight heparin. Among the patients with RC, 41/61 (67.2%) required ICU care and 50/61 (81.9%) had mean oxygen saturation levels of 85% on day 9 of the illness. Treatment for these patients comprised high-flow oxygen for 31/61 (50.8%), continuous positive airway pressure (CPAP) for 14/61 (22.9%) and invasive ventilation for 5/61 (8.2%).
Intravenous antibiotics were given to patients with elevated procalcitonin, positive sputum or blood culture and/or elevated CRP in whom concurrent bacterial infection was suspected. Tocilizumab was administered to patients who had the following parameters: ferritin ≥600 ng/mL, CRP ≥80 mg/L32 and IL-6 >37.65 pg/ml.33 We recorded 10 (16.4%) deaths during the study period, all of which occurred among the RC group. Two or more comorbidities were reported in 39/61 (63.9%) patients with RC, in contrast to the non-respiratory-compromised group, where 51/57 (89.5%) had only one or no comorbidities. The demographic and clinical characteristics of the study cohort as well as details of treatment are summarized in Table 1.
Table 1.
Demographic characteristics of the study population
| Variable | n (%) |
|---|---|
| Age (years) | |
| < 30 | 15 (12.7) |
| 31–40 | 20 (16.9) |
| 41–50 | 21 (17.8) |
| 51–60 | 25 (21.2) |
| 61–70 | 29 (24.6) |
| ≥71 | 8 (6.8) |
| Gender | |
| Male | 63 (53.4) |
| Female | 55 (46.6) |
| Comorbidities | |
| Hypertension | 37 (31.3) |
| Diabetes | 30 (25.4) |
| Dyslipidaemia | 12 (10.2) |
| Ischaemic heart disease | 9 (7.6) |
| Chronic kidney disease | 8 (6.7) |
| Chronic liver disease | 8 (6.7) |
| Asthma | 6 (5.1) |
| COPD | 5 (4.2) |
| None | 59 (50.0) |
| Days following onset of symptoms | |
| 3 | 96 (81.3) |
| 4 | 18 (15.2) |
| 5 | 4 (3.4) |
| Length of hospital stay | |
| <14 | 75 (63.5) |
| 14–16 | 37 (31.4) |
| ≥17 | 6 (5.1) |
| Symptoms | |
| Fever | 84 (71.2) |
| Headache | 54 (45.8) |
| Cough | 93 (78.8) |
| Dyspnoea | 61 (51.7) |
| Arthralgia/myalgia | 84 (71.2) |
| Diarrhoea | 28 (23.7) |
| Sore throat | 78 (66.1) |
| Setting of care | |
| Non-respiratory compromised (n=57 [48.3%]) | |
| General ward | 57 (100) |
| ICU | 0 (0) |
| Respiratory compromised (n=61 [51.7%]) | |
| General ward | 20 (32.8) |
| ICU | 41 (67.2) |
| Mode of oxygen therapy for respiratory-compromised patients (n=61) | |
| Face mask | 11 (18.1) |
| High flow | 31 (50.8) |
| CPAP | 14 (22.9) |
| Invasive ventilation | 5 (8.2) |
| Medication management for respiratory-compromised patients (n=61) | |
| Intravenous dexamethasone 6 mg daily for a median of 10 d | 61 (100) |
| Two doses of intravenous tocilizumab (median dose of 400 mg daily) | 46 (75.4) |
| Intravenous antibiotic | 61 (100) |
| Subcutaneous low molecular heparin (median dose of 40 mg daily) | 61 (100) |
| Oral azithromycin 500 mg daily | 61 (100) |
| Outcome | |
| Non respiratory compromised (n=57) | |
| Recovered | 5 7(100) |
| Death | 0 (0) |
| Respiratory compromised (n=61) | |
| Recovered | 51 (83.4) |
| Death | 10 (16.4) |
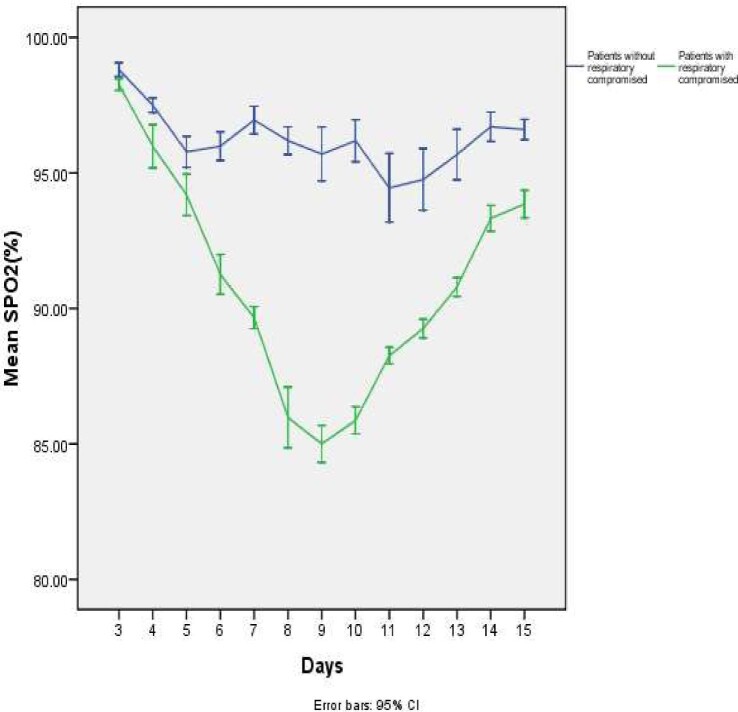
Respiratory rate on admission (19.87±2.87 vs 17.12±1.41; p=0.0001) and length of hospital stay (14.21±1.53 vs 10.51±1.85; p<0.0001) were significantly higher in the RC group. There was a statistically significant difference in oxygen saturation levels between patients with and without RC from day 3 onwards. Figure 1 shows the variation of oxygen saturation by pulse oximeter in patients with and without RC. Table 2 shows the demographic characteristics, admission vital signs and vaccination status of patients with and without RC. All patients in the RC group had CXR abnormalities, including ground glass opacities, consolidation or effusions. Table 3 summarizes the laboratory and radiological findings of patients with and without RC.
Figure 1.
Variation of oxygen levels by pulse oximetry (on room air) of patients with and without respiratory compromise.
Table 2.
Comparison of demographic characteristics, vital signs on admission and vaccination status of patients with and without respiratory compromise
| Variable | Patients with respiratory compromise (n=61) | Patients without respiratory compromise (n=57) | p-Value |
|---|---|---|---|
| Age (years), mean±SD | 51.30±14.48 | 49.07±15.18 | 0.427 |
| Gender, n (%) | |||
| Male | 36 (59.1) | 27 (48.9) | 0.686 |
| Female | 25 (40.9) | 30 (53.1) | |
| Comorbidities, n (%) | |||
| 0 | 7 (11.5) | 37 (64.9) | <0.0001 |
| 1 | 15 (24.6) | 14 (24.6) | |
| 2 | 23 (37.7) | 3 (5.3) | |
| 3 | 16 (26.2) | 3 (5.3) | |
| Length of hospital stay (days), mean±SD | 14.21±1.53 | 10.51±1.85 | <0.0001 |
| Systolic blood pressure median day 3 of illness (mmHg), mean±SD | 127.51±10.64 | 132.12±13.32 | 0.065 |
| Diastolic blood pressure median day 3 of illness (mmHg), mean±SD | 75.98±5.98 | 78.69±5.95 | 0.028 |
| Heart rate/min median day 3 of illness, mean±SD | 85.85±9.83 | 86.43±8.64 | 0.76 |
| Respiratory rate/min median day 3 of illness, mean±SD | 19.87±2.87 | 17.12±1.41 | <0.0001 |
| Vaccination statusa | |||
| None | 50 (81.9) | 21 (36.8) | <0.0001 |
| 1 dose | 10 (16.4) | 36 (63.2) | |
| 2 doses | 1 (1.7) | 0 (0) |
Oxford-AstraZeneca in all patients.
Significant values in bold.
Table 3.
Comparison of laboratory investigations, radiological findings and Ct among patients with and without respiratory compromise (median day 3 of illness)
| Variable | Patients with respiratory compromise (n=61) | Patients without respiratory compromise (n=57) | p-Value |
|---|---|---|---|
| White cell count (109/L), mean±SD | 2.73±1.23 | 3.36±1.78 | 0.048 |
| Platelet count (109/L), mean±SD | 142.2±35.37 | 145.63±35.62 | 0.640 |
| Lymphocyte count (%), mean±SD | 18.64±3.42 | 28.78±10.96 | <0.0001 |
| Absolute lymphocyte count (103/μL), mean±SD | 531.49±289.09 | 764.69±481.79 | 0.001 |
| Absolute neutrophil count (103/μL), mean±SD | 1818.61±185.34 | 1989.34±278.32 | <0.0001 |
| Neutrophils (%) | 66.5 | 58.3 | 0.359 |
| NLR, mean±SD | 3.42±0.75 | 2.59±0.55 | 0.001 |
| Atypical lymphocyte count (109/L), mean±SD | 0.18±0.11 | 0.24±0.12 | 0.328 |
| C-reactive protein (mg/L), mean±SD | 31.98±22.84 | 30.29±19.49 | 0.944 |
| ALT (units/L), mean±SD | 95.06±101.44 | 92.76±106.05 | 0.910 |
| AST (units/L), mean±SD | 142.62±149.85 | 153.47±192.89 | 0.875 |
| PCV (%), mean±SD | 38.80±4.94 | 40.08±4.46 | 0.186 |
| Blood group, n (%) | |||
| A | 13 (21.3) | 17 (29.8) | 0.737 |
| B | 16 (26.2) | 14 (24.6) | |
| O | 26 (42.6) | 22 (38.6) | |
| AB | 6 (9.8) | 4 (7.0) | |
| CXR findings, n (%) | |||
| Ground glass opacities | 61 (100.0) | 0 (0) | N/A |
| Peripheral consolidations | 21 (34.42) | 1 (1.75) | <0.0001 |
| Effusion | 16 (26.22) | 2 (3.5) | <0.0001 |
| Ct, mean±SD | 19.46±2.64 | 22.62±3.37 | <0.0001 |
ALT: alanine transaminase, AST: aspartate aminotransferase, PCV: packed cell volume.
Significant values in bold.
While the AL on admission was significantly lower (531.49±289.09×103/μL vs 764.69±481.79×103/μL; p=0.0001), the NLR was significantly higher (3.42±0.75 vs 2.59±0.55; p=0.0001) in the group with RC. The Ct value was also significantly lower in the RC group (19.46±2.64 vs 22.62±3.37; p=0.0001). ROC analysis demonstrated that a Ct value <19.9, AL <630.8×103/μL and NLR >3.12 were predictive of hypoxia (oxygen saturation on air by pulse oximeter <92%) on day 7 of illness. The Ct, AL and NLR thresholds when used as a combined modal showed a stronger predictive power for hypoxia on day 7 of illness than when used individually (AUC 0.805, sensitivity 96.7%, specificity 69.1%). These data are summarized in Figure 2. The Ministry of Health guidelines in Sri Lanka define critical or severe COVID-19 based on oxygen saturation <92%. In our cohort, the mean oxygen saturation was 92% before day 7 and 90% on or after day 7. We therefore chose day 7 as the time point at which we sought to predict hypoxia.
Figure 2.
The Ct value, AL count and NLR on day 3 of illness as predictors of oxygen saturation <92% on day 7 of illness.
Discussion
We demonstrate for the first time that the combination of NLR, AL and Ct on day 3 of COVID-19 illness is predictive of hypoxia on day 7 of disease. While Ct values <20 at diagnosis were proposed as a marker of severity of COVID-19, this has not been consistently demonstrated across studies.34,35 However, the Ct values proposed by other groups as predictive of severe disease are similar to those we identified.36,37 Importantly, none of these studies looked at the combined predictive power of the Ct value and haematologic parameters.
Lymphopenia has been established as a feature of severe COVID-19 and a predictor of disease severity.25,38,39 The predictive AL cut-off varies between studies, with values ranging from <950×103/μL to <600×103/μL.40–44 These findings were confirmed in a systemic review and meta-analysis showing that COVID-19 patients with a good outcome had a significantly higher AL on admission than those with a poor outcome (mean difference between groups was 361.06×103/μL).22 The AL values proposed by these studies are similar to those we report in this study. While a high lymphocyte:CRP ratio has also been proposed as an adverse prognostic factor in COVID-19, the non-specific nature of CRP may affect the routine applicability of this parameter.45
High NLR values have been reported in severe COVID-19, with thresholds of 5,28 3.3 and 4.7 being proposed as predictors of severity.40 These values are similar to what we have proposed as predictive in our cohort and were also shown to be predictive at day 3 of symptoms. The similarity of predictive AL and NLR values between published data and ours suggests that the haematologic parameters in South Asian COVID-19 patients are similar to those of their East Asian counterparts.25
The mean age of patients with RC in our study (51.30±14.48 y) was similar to that reported by other groups.41 We demonstrated that the patients with more than two comorbidities were at higher risk of RC, which is in keeping with results from other countries.41–43 In contrast to other reports from Asia, we found no gender disparity in COVID-19 severity within our cohort.46 In keeping with published data, we demonstrated that vaccinated individuals appear to have less severe COVID-19 disease, however, the relatively small numbers preclude a definitive comparison.47,48 There is a paucity of data on the timing of hypoxia in COVID-19 patients. We found that severe hypoxia, defined by oxygen saturation <92%, occurred most commonly on day 7 of illness. These findings need to be validated in independent cohorts.
Limitations of our study include the fact that it was a retrospective analysis conducted at a single centre in Sri Lanka. Our findings need to be validated prospectively in larger independent cohorts within and outside South Asia. Future studies are required evaluating these parameters in conjunction with Ct, AL and NLR values as predictive tools for severe COVID-19. With expanding vaccination programs across the globe, how these predictive thresholds apply to fully vaccinated cohorts of patients would also be an important area to study.
Conclusions
We propose that a Ct value <19.9, AL <630.8×103/μL and NLR >3.12 on day 3 of symptoms is predictive for hypoxia on day 7 of COVID-19 illness. These are readily available parameters that can be used routinely in developing countries in a pandemic setting. The results of our study are of clinical relevance to clinicians managing COVID-19 in resource-limited settings, where this combinations of parameters may provide a low-cost means of triaging patients.
Supplementary Material
Contributor Information
Visula Abeysuriya, Nawaloka Hospital Research and Education Foundation, Nawaloka Hospitals PLC, Colombo, Sri Lanka.
Suranjith L Seneviratne, Nawaloka Hospital Research and Education Foundation, Nawaloka Hospitals PLC, Colombo, Sri Lanka; Institute of Immunity and Transplantation, Royal Free Hospital and University College London, UK.
Arjuna P de Silva, Department of Medicine, Faculty of Medicine, University of Kelaniya, Sri Lanka.
Riaz Mowjood, Department of Respiratory Disease, Nawaloka Hospitals PLC, Colombo, Sri Lanka.
Shazli Mowjood, Department of Respiratory Disease, Nawaloka Hospitals PLC, Colombo, Sri Lanka.
Thushara de Silva, Department of Respiratory Disease, Nawaloka Hospitals PLC, Colombo, Sri Lanka.
Primesh de Mel, Nawaloka Hospital Research and Education Foundation, Nawaloka Hospitals PLC, Colombo, Sri Lanka.
Chandima de Mel, Nawaloka Hospital Research and Education Foundation, Nawaloka Hospitals PLC, Colombo, Sri Lanka.
Lal Chandrasena, Nawaloka Hospital Research and Education Foundation, Nawaloka Hospitals PLC, Colombo, Sri Lanka.
R S Wijesinha, Princess Alexandra Hospital, Princess Alexandra Hospital NHS Trust, UK.
Amitha Fernando, National Hospital, Colombo, Sri Lanka.
Sanjay de Mel, Department of Haematology-Oncology, National University Cancer Institute, National University Health System Singapore, Singapore.
Authors’ contributions
VA, SDM, SLS, APS, RM, TS, AF, CDM and LG conceptualized the study. VA, RSW, SM and PDM collected the data. VA, SDM, SLS and RM analysed the data and wrote the manuscript. VA, LC, CDM, SLS, AF and SDM conducted critical review and editing of manuscript. All authors read and approved the final manuscript. VA and SDM are guarantors of the paper.
Acknowledgements
We acknowledge the assistance given by the Director/General Manager and the management of Nawaloka Hospital, Colombo, Sri Lanka. We also thank the staff of the Medical Records Office and Computer Division of Nawaloka Hospital. Finally, we thank all patients and next-of-kin for providing the necessary information required for the study.
Funding
None.
Competing interests
None declared.
Ethical approval
Ethical approval for this study was obtained from the Ethics Review Committee of Nawaloka Hospital, Colombo, Sri Lanka.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. World Health Organization . Rolling updates on coronavirus (COVID-19). WHO characterizes COVID-19 as a pandemic, 2019–20. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen [accessed 2 December 2021].
- 2. Jayasekara D, Seneviratne SL, Jayasekara Aet al. . Atypical presentations of COVID-19. Adv Infect Dis. 2020;10(3):136–42. [Google Scholar]
- 3. Rahman A, Niloofa R, De Zoysa IMet al. . Neurological manifestations in COVID-19: a narrative review. SAGE Open Med. 2020;8:205031212095792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kariyawasam JC, Jayarajah U, Riza Ret al. . Gastrointestinal manifestations in COVID-19. Trans R Soc Trop Med Hyg. 2021;doi: 10.1093/trstmh/trab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rahman A, Tabassum T, Araf Yet al. . Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol Biol Rep. 2021;48(4):3863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen N, Zhou M, Dong Xet al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu Cet al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan W, Ni Z, Hu Yet al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu XW, Wu XX, Jiang XGet al. . Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ministry of Health Sri Lanka . Provisional clinical practice guidelines on COVID-19 suspected and confirmed cases. Colombo: Ministry of Health Sri Lanka; 2020. [Google Scholar]
- 12. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JMet al. . SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Yang Y, Zhang Cet al. . Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan C, Zhu H, Yang Yet al. . Viral loads in throat and anal swabs in children infected with SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):1233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li P, Wu W, Zhang Tet al. . Implications of cardiac markers in risk-stratification and management for COVID-19 patients. Crit Care. 2021;25:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang Y-W, Schmitz JE, Persing DHet al. . Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58(6):e00512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bustin SA, Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci. 2005;109:365–79. [DOI] [PubMed] [Google Scholar]
- 18. Rao SN, Manissero D, Steele VRet al. . A narrative systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020;9(3):573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazzoni A, Salvati L, Maggi Let al. . Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020;130(9):4694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiong Y, Liu Y, Cao Let al. . Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu C, Liu Y, Chen Bet al. . Prognostic value of lymphocyte count in severe COVID-19 patients with corticosteroid treatment. Signal Transduc Target Ther. 2021;6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bordon J, Aliberti S, Fernandez-Botran Ret al. . Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis. 2013;17(2):e76–83. [DOI] [PubMed] [Google Scholar]
- 24. Borges L, Pithon-Curi TC, Curi Ret al. . COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020:8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahman A, Niloofa R, Jayarajah Uet al. . Hematological abnormalities in COVID-19: a narrative review. Am J Trop Med Hyg. 2021;104(4):1188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dennison D, Al Khabori M, Al Mamari Set al. . Circulating activated neutrophils in COVID-19: an independent predictor for mechanical ventilation and death. Int J Infect Dis. 2021;106:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imran MM, Ahmad U, Usman Uet al. . Neutrophil/lymphocyte ratio—a marker of COVID-19 pneumonia severity. Int J Clin Pract. 2021;75(4):e13698. [DOI] [PubMed] [Google Scholar]
- 28. Liu L, Zheng Y, Cai Let al. . Neutrophil-to-lymphocyte ratio, a critical predictor for assessment of disease severity in patients with COVID-19. Int J Lab Hematol. 2021;43(2):329–35. [DOI] [PubMed] [Google Scholar]
- 29. AccuPower® SARS-CoV-2 Real-Time RT-PCR Kit. Available from: https://us.bioneer.com/pagecat1/diagnostic/AccuPower-SARS-CoV2-RealTime-RT-PCR-Kit [accessed 2 December 2021].
- 30. RealStar® SARS-CoV-2 RT-PCR Kit. Available from: https://altona-diagnostics.com/en/products/reagents-140/reagents/realstar-real-time-pcr-reagents/realstar-sars-cov-2-rt-pcr-kit.html [accessed 2 December 2021].
- 31. World Health Organization . COVID-19 clinical management: living guidance. Geneva: World Health Organization; 2021. [Google Scholar]
- 32. Meleveedu KS, Miskovsky J, Meharg Jet al. . Tocilizumab for severe COVID-19 related illness – a community academic medical center experience. Cytokine X. 2020;2(4):100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Hao Y, Ou Wet al. . Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med. 2020;18:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trunfio M, Venuti F, Alladio Fet al. . Diagnostic SARS-CoV-2 cycle threshold value predicts disease severity, survival, and six-month sequelae in COVID-19 symptomatic patients. Viruses. 2021;13(2):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah S, Singhal T, Davar Net al. . No correlation between Ct values and severity of disease or mortality in patients with COVID 19 disease. Indian J Med Microbiol. 2021;39(1):116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jefferson T, Spencer EA, Brassey Jet al. . Viral cultures for COVID-19 infectious potential assessment – a systematic review. Clin Infect Dis. 2020;doi: 10.1093/cid/ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukushima T, Kabata H, Yamamoto Ret al. . The real-time reverse transcription-polymerase chain reaction threshold cycle values for severe acute respiratory syndrome coronavirus 2 predict the prognosis of coronavirus disease 2019 pneumonia. Respir Investig. 2021;59(3):360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang F, Nie J, Wang Het al. . Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henry BM, de Oliveira MHS, Benoit Set al. . Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–8. [DOI] [PubMed] [Google Scholar]
- 40. Yang A-P, Liu J, Tao Wet al. . The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu X, Yu C, Qu Jet al. . Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grasselli G, Greco M, Zanella Aet al. . Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xia X, Wen M, Zhan Set al. [An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19]. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(3):333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bal T, Dogan S, Cabalak Met al. . Lymphocyte-to-C-reactive protein ratio may serve as an effective biomarker to determine COVID-19 disease severity. Turk J Biochem. 2020;46(1):21–6. [Google Scholar]
- 46. Gausman J, Langer A.. Sex and gender disparities in the COVID-19 pandemic. J Womens Health. 2020;29(4):465–6. [DOI] [PubMed] [Google Scholar]
- 47. Good Morning Britain . Covid vaccines reduce the likelihood of hospitalisation by 80% after one dose in over 70s, data shows. Available from: https://www.itv.com/goodmorningbritain/articles/covid-vaccines-reduce-the-likelihood-of-hospitalisation-by-80-percent-data[accessed 2 December 2021].
- 48. Seneviratne SL, Jayarajah U, Abeysuriya Vet al. . COVID-19 vaccine landscape. J Ceylon Coll Physicians. 2020;51(2):120–131. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.