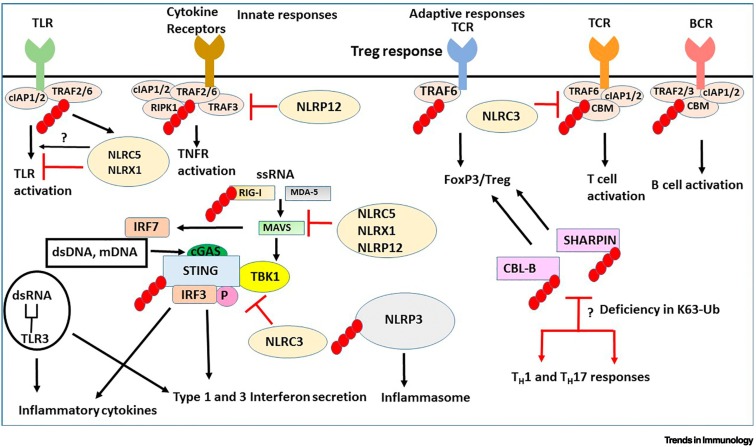
Figure 3.
Ubiquitination signaling pathways in innate and adaptive immunity.
The black arrows indicate stimulation of a pathway and the red arrows denote either inhibition or functional implications due to inhibition. The small red ovals attached to proteins are K63-linked ubiquitin chains. Membrane Toll-like receptors (TLRs) and cytokine receptors [including canonical tumor necrosis factor (TNF) receptors] activate ubiquitinated TNF receptor-associated factor 2/6 (TRAF2/6) pathways to stimulate cytokine and interferon (IFN) production. Single-strand (ss)RNA, double-strand (ds)RNA and mitochondrial (mt)DNA activate cytosolic receptors that are also controlled by ubiquitination. Stimulator of interferon genes (STING) is an intermediate for activation of downstream IFN-activating genes, IFN regulatory factor 3/7 (IRF3/7) by retinoic acid inducible gene-I (RIG-I), mitochondrial antiviral-signaling protein (MAVS), melanoma differentiation-associated protein 5 (MDA-5) through TANK-binding kinase 1 (TBK1), cyclic GMP-AMP (cGAMP) sensing dsRNA. K63 ubiquitination of SHANK-associated RH domain interacting protein in postsynaptic density (SHARPIN) and Casitas B-lineage Lymphoma-b (CBL-B) promotes regulatory T cell (Treg) lineage commitment and deficiency in K63-ubiquitinated SHARPIN or CBL-B stimulates helper T cell (TH) 1 and TH17 inflammatory responses. K63 ubiquitination of NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) has a role in the activation of the inflammasome. Nod-like receptor card domain 3 (NLRC3) blocks both STING activation and TNF receptor-associated factor 6 (TRAF6) activation. NLRC5 and NLRX1 inhibit IKK activation, enhancement of nuclear factor KappaB (NF-κB) and inflammation signaling by TLRs. NLRP12 negatively regulates non-canonical NF-κB pathway. NLRC5, NLRX1, and NLRP12 negatively regulate RIG-induced antiviral immune responses. Inhibition of NF-κB, RIG-I, and MDA-5 signaling (by ssRNA) is blocked when NLRC5 is K63-ubiquitinated.