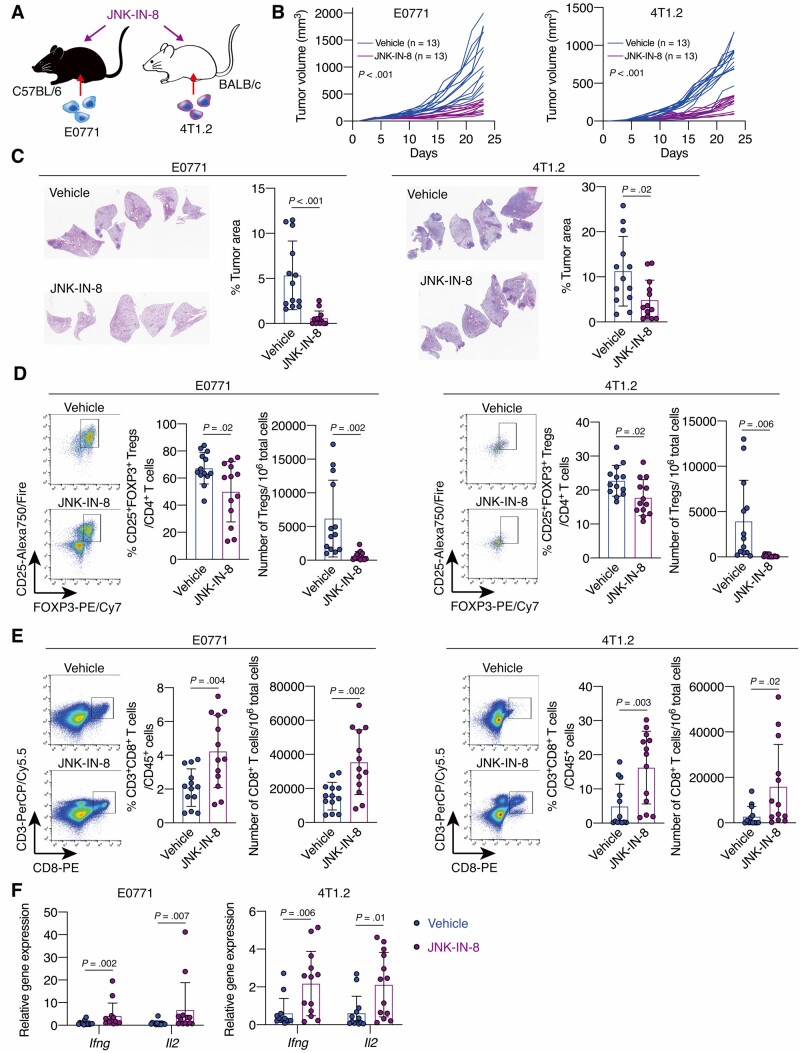
Figure 2.
The effect of C-JUN N-terminal kinase (JNK) inhibitor treatment on triple-negative breast cancer tumor growth, metastasis, and the immune tumor microenvironment. A) Schematic showing the experimental design: mouse breast cancer E0771 or 4T1.2 cells were injected into C57BL/6 or BALB/c mice, respectively. Mice were treated with JNK-IN-8 when the tumors became palpable. B) Tumor growth curves for vehicle- or JNK-IN-8–treated E0771 (n = 13 per group) and 4T1.2 (n = 13 per group) mouse models. C) Hematoxylin-eosin staining and quantification of the percentage tumor area for the lungs of vehicle- or JNK-IN-8–treated E0771 (n = 13 per group) and 4T1.2 (n = 13 per group) mice. D and E) Flow cytometry analysis for D) regulatory T cells and E) cluster of differentiation 8(CD8+)T cells in tumors from vehicle- or JNK-IN-8–treated E0771 (n = 13 per group) and 4T1.2 (n = 13 per group) mouse models. LIVE/DEAD Aqua– and CD45+ cells were gated. Shown are quantification of D) the percentage of CD25+ forkhead box p3 (FOXP3+) cells in CD3+CD4+ cells and the number of CD3+CD4+CD25+FOXP3+ cells in 106 tumor cells and E) the percentage of CD3+CD8+ cells in CD45+ cells and the number of CD3+CD8+ cells in 106 tumor cells. F) Ifng and Il2 mRNA expression in tumors from vehicle- or JNK-IN-8–treated E0771 (n = 13 per group) and 4T1.2 (n = 13 per group) models were evaluated by quantitative reverse transcription-polymerase chain reaction. Data are summarized as mean and error bars represent the SD. The Wilcoxon 2-sample test (B) and a 2-tailed Student t test were used to calculate P values. All statistical tests were 2-sided.