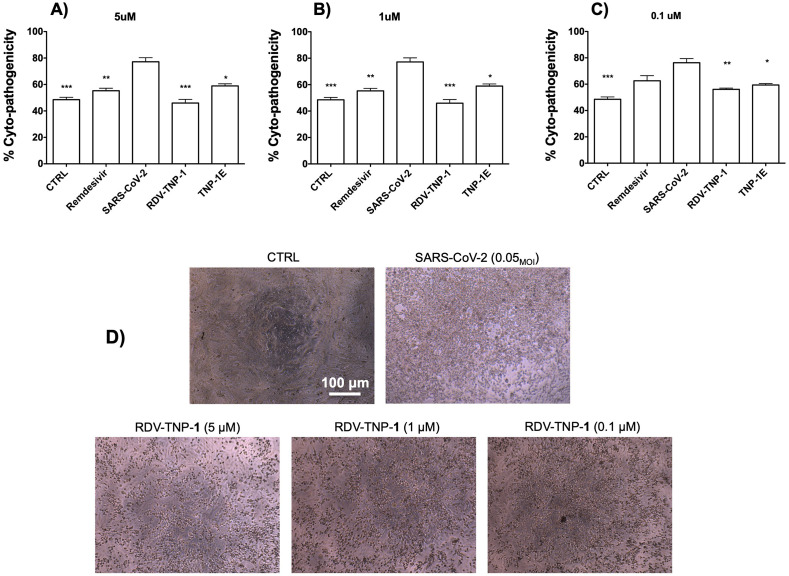
Fig. 6.
Protection against SARS-CoV-2 induced cytopatogenic effect by TNP-1 treatment. (A-C) Cells were evaluated at different concentrations (5, 1, 0.1 μM) of RDV-loaded and nonloaded NP, using SARS-CoV-2 and RDV as controls. Empty (E) NP were tested at the same NP w/v concentration (μg/mL) of the parent RDV-loaded nanosample (300, 60, 6 μg/mL, for TNP-1E, which correspond to 5, 1, 0.1 μM, for RDV-TNP-1, respectively). Vero E6 cells were treated with samples and infected with SARS-CoV-2 at 0.05MOI. At 72 h post infection, inhibition of SARS-CoV-2-induced CPE was determined as survival of infected or not infected cells, and measured by using AB assay. The results were evaluated setting the uninfected control cells as 100% viability and the remaining values represented as a relative value. Data are mean ± SD, n = 6 replicates. (D) Representative microscopic images showing inhibition of virus-induced CPE in Vero E6 cells. From left to right: not infected Vero E6 cell monolayer (complete absence of CPE) as a control (CTRL), SARS-CoV-2 infected cells at MOI of 0.05, after 72 h post infection, and SARS-CoV-2 infected Vero E6 after RDV-TNP-1 exposure at different concentrations (5, 1, 0.1 μM). (∗), (∗∗) and (∗∗∗) significantly (∗∗∗p < 0.001, ∗∗p < 0.01 and ∗p < 0.05vs. C6-NP-0, n = 4) by one-way ANOVA and post-hoc Dunnett's test.