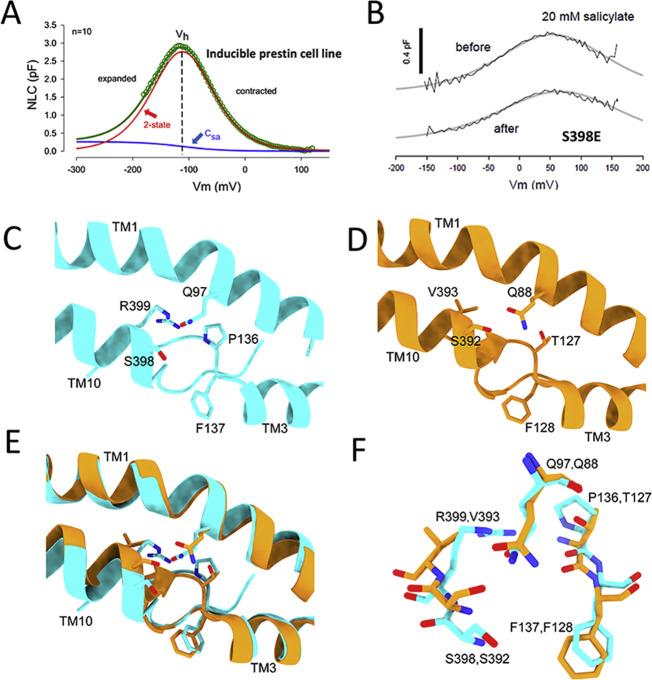
Fig. 3. Nonlinear capacitance represents prestin voltage dependent activity and does not depend on anion binding.
A Prestin predominantly resides in the contracted state at 0 mV. Average NLC (n = 10), 48 h after induction of our inducible prestin HEK stable cell line (#C16). Recordings were done in the presence of 140 mM intracellular Cl. NLC-fitted (Eq. 1) parameters were Qmax = 0.40; z = 0.70; Vh = −112 mV; DCsa = 0.26 pF; NLC peaks at Vh; n = 10. B The S398E mutation in prestin preserves NLC after application of 20 mM salicylate. Controls shows full block of NLC. The average unitary gating charge (z) in these mutants (0.69e ± 0.03 SEM, n = 7) was similar to that of CHO cells expressing prestin–YFP (0.73e ± 0.14 SEM, n = 10, P > 0.05). Vh was significantly different, however (96.25 mV± 4.6 WT; +73.8mV ±6.4, p < 0.05). C Close-up view showing the residues Q97, S398, R399, P136, and F137 in prestin (displayed as sticks, colored by atom type) that correspond to residues found to be important for Cl− binding in Slc26a9 (with prestin in cyan ribbon). The mean z and Vh values of fitted NLC of several mutations of residues that coordinate chloride binding were WT, 0.71e ± 0.03 SEM, −98.12 mV ± 2.33SEM n = 9; Q97A 0.65 ± 0.04, −94.1 mV ± 5.29, n = 5; P136T 0.64 ± 0.05 SEM, −88.15 ± 10.1 SEM, n = 9. The differences were not significant (p = 0.54, one-way ANOVA for z; p = 0.605, one-way ANOVA for Vh). D Close-up view of the corresponding region in the Slc26a9 structure (the “inward-facing”, open conformation, in orange ribbon). Residues Q88, S392, T127, and F128 (in stick representation) are involved in Cl-coordination in Slc26a9. E The location of the Cl binding site at the interface between TM1, TM3, and TM10 is conserved in prestin and Slc26a9. Overlay of portions of the transmembrane helices 1, 3, and 10, from prestin and Slc26a9, as shown in panels (C, D). F Overlay of the Cl-binding sites of prestin and Slc26a9 (“inward-facing”, open conformation). Equivalent residues are shown as pairs. Cyan sticks are prestin residues and orange sticks are Slc26a9 residues. Source data are provided as a Source Data file.