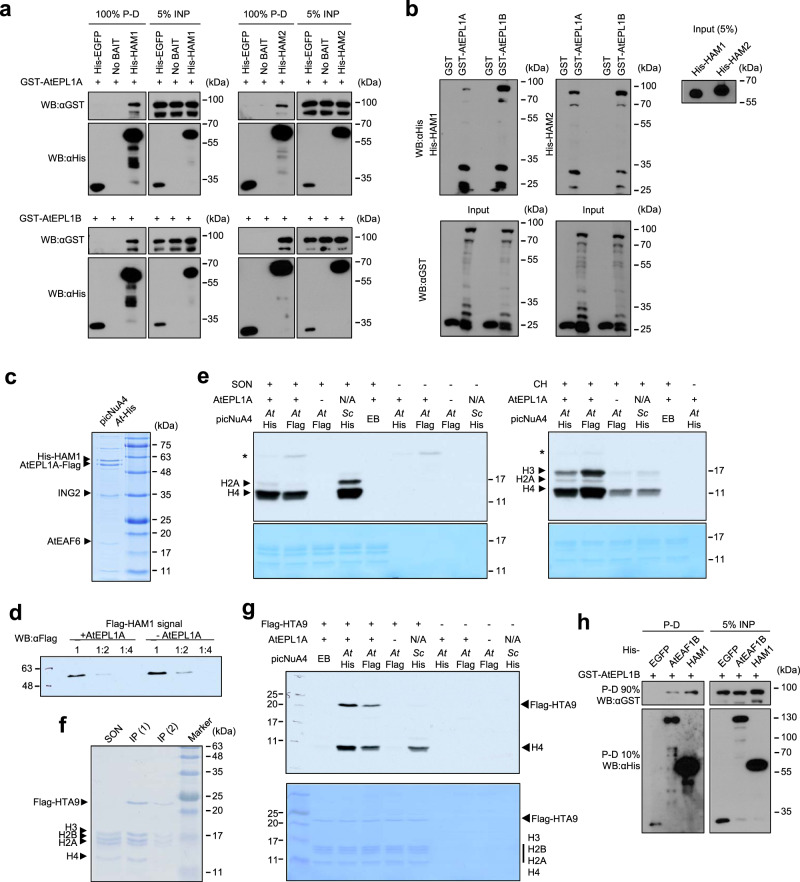
Fig. 4. AtEPL1 interacts directly with the catalytic and platform subunits of Arabidopsis NuA4 and supports nucleosomal H4 and H2A.Z acetylation in vitro.
a Pulldown assay showing the interaction between the recombinant AtEPL1 and HAM paralog pairs expressed in bacteria. Pull-down and input are abbreviated P-D and INP, respectively. b Interactions between AtEPL1s and HAMs were analyzed by far western blotting. After SDS–PAGE and western blotting of free GST and GST-AtEPL1A/B, the upper membranes were sequentially incubated with His-HAM1/2 and anti-His antibodies, whereas the lower membranes were incubated with an anti-GST antibody. The lower bands in the upper panels most likely indicate the interaction of HAM1/2 with partially degraded AtEPL1B. c Purification of recombinant A. thaliana picNuA4 from bacteria (Coomassie staining). A 6×His tag fused to HAM1 was used for purification on cobalt affinity resin. The numbers in the brackets indicate the expected molecular weights of the recombinant picNuA4 subunits. d Western blot showing Flag-HAM1 normalized protein levels in purified fractions from bacteria producing At picNuA4 complexes with and without AtEPL1A. Both fractions retained the Flag-HAM1 signal after ING2-His purification, indicating that AtEPL1A is not required for HAM1 binding. e In vitro assay of the HAT activity of recombinant A. thaliana (At) picNuA4 toward short oligonucleosomes (SONs) and free core histones (CHs). Recombinant S. cerevisiae (Sc) picNuA4 was used as a positive control. At-His corresponds to the purified His-HAM1 complex, and At-Flag indicates two versions of the Flag-HAM1-purified complex (see Supplementary Fig. 8a), with and without AtEPL1A, as shown in d. Autoacetylation on AtEAF6 is highlighted with an asterisk. f Immunoprecipitation (IP) of Flag-conjugated Arabidopsis H2A.Z protein (Flag-HTA9) from chromatin extract resulted in the isolation of human nucleosomes containing Arabidopsis H2A.Z. IP (1) and IP (2) correspond to Flag peptide eluates 1 and 2, respectively. SONs were used as a control. g In vitro assay of the HAT activity of recombinant A. thaliana picNuA4 toward Arabidopsis H2A.Z histone (FLAG-HTA9)-containing oligonucleosomes. Three versions of At picNuA4 were used, similar to the procedure in f. Only complexes containing AtEPL1A efficiently acetylated nucleosomal HTA9. Sc picNuA4 was used as a control with no activity towards HTA9. h Pull-down assay showing the interaction between AtEPL1B and a fragment of AtEAF1B corresponding to AAs 430-1322. HAM1 was used for comparison. All proteins were expressed in bacteria as either GST or 6×His fusions. Source data are provided as a Source Data file.