Summary
Background
Reliable vascular access is key to sustainable haemodialysis treatment. Guidelines recommend an arteriovenous fistula (AVF) as the preferred modality in preference to arteriovenous grafts (AVGs) or central venous catheters (CVCs). There are limited data on vascular access in sub- Saharan Africa. This study aimed to evaluate the vascular access used in a South African tertiary hospital and identify problems with achieving the recommended access goals.
Methods
A cross-sectional analysis was performed of the haemodialysis programme at Livingstone Tertiary Hospital. Current and initial vascular access used, timing until the creation of permanent access, and any complications experienced were recorded.
Results
CVCs were used in 56% of subjects, 38% were using an AVF and 5% were using an AVG. Only 12% of the group had no AVF attempt. The overwhelming majority (95%) had dialysis initiated with a CVC. The rate of pre-emptive AVF creation was low and a delay in AVF creation was seen in 63% of patients. Central venous stenosis or occlusion was present in 26% of patients and likely due to prior or current CVC use.
Conclusions
The prevalence of CVC use was high and there were significant delays to AVF creation. High rates of central venous stenosis compromise future AVF use and are likely due to prolonged CVC use. Changes needed to improve the vascular access service include a multidisciplinary access clinic, dedicated theatre list, vascular access co-ordinator and further data collection to continually evaluate the vascular access service.
Keywords: arteriovenous fistula, haemodialysis access, arteriovenous graft, tunnelled central venous catheter
Haemodialysis offers life-saving therapy to patients with advanced chronic kidney disease (CKD). Unfortunately, access to haemodialysis is limited by cost, availability and reliable vascular access.1 Optimal management of this limited resource is therefore of key importance in reducing the high burden placed on the healthcare system.2-4
Currently, vascular access options are limited to an autogenous arteriovenous fistula (AVF), a prosthetic arteriovenous graft (AVG) and a central venous catheter (CVC). It is well established that the autogenous AVF is superior to the other modalities in terms of patency rates and infection risk. This is reflected in local and international guidelines where it is recommended as the primary option for all patients on haemodialysis.5-7 The Fistula First Breakthrough Initiative and the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines in 2006 set in motion a drive to create more AVFs and limit the use of CVCs.7 This saw a change in practice in high-income countries as more fistulae were created and fewer AVGs and CVCs were used.6
Access complications contribute to ineffective dialysis and interruptions in treatment, which further contribute to the cost of care. Significant problems with AVFs include non-maturation and early thrombosis. Some studies have shown an early failure rate as high as 46%.6 If no suitable vessels are available, or when all vessels in the arm are exhausted, a prosthetic AVG can be placed.8 The risk of infection is increased for an AVG compared to an autogenous AVF but primary patency may be higher.7 The most common problem with an AVG is stenosis due to an abnormal turbulent flow pattern, which causes focal shear stress in the native blood vessel and neo-intimal hyperplasia, which ultimately leads to narrowing and thrombosis of the graft.9
CVCs are the least-preferred modality and not recommended for permanent access.5 They have a high infection risk and also cause permanent damage to the native vessels, which can lead to central venous stenosis and occlusion, eventually limiting future access modalities. They do have some benefits however, since they can reliably be used as soon as they are placed when urgent dialysis is required. CVCs also cause less haemodynamic change and no increase in blood flow to the heart, which may be important in patients with congestive cardiac failure.10
The choice of vascular access should be individualised according to the specific patient characteristics. The primary goal should be a distal autogenous AVF in the non-dominant arm, created three to six months prior to the expected start of haemodialysis.5,6 This would allow time for maturation and even intervention in the event the fistula fails to mature adequately, which will decrease the need for CVC use. A recent study suggested that the benefit of an autogenous fistula is lost when a patient is started on a CVC and then has an AVF created.11 The use of CVCs, even for a short period, should be discouraged. This practice relies on the timeous identification and referral of patients with CKD.
Reports from middle- and low-income countries show a common theme: a high rate of AVF creation but typically only after initiating dialysis with a CVC (90 to 95%).12,13 Factors associated with improved outcomes in these countries include early referral and a multidisciplinary approach.14,15 Late referral, conduit damage by venepuncture and a lack of secondary intervention for failing fistulae contributes to high failure rates.16
Another strategy proven to improve outcomes includes pre-operative ultrasound to evaluate the size and quality of the vein to be used.6,17 A structured pre-dialysis care programme allows the patient to be adequately prepared with counselling and training, as well as early referral to the vascular access surgeon.17 Regular multidisciplinary meetings are useful to refer new patients, discuss patients with early concerns about access complications and deal with problematic vascular access.17 Having a dedicated vascular access co-ordinator with a pre-operative ultrasound protocol was shown to be the most important factor in improving haemodialysis access outcomes.18
There is currently no database for vascular access in South Africa, and high-quality data in low- and middle-income countries are limited.4 The South African Renal Registry was the only active African registry until the establishment of the African Renal Registry in 2015.19 Unfortunately, they do not yet record vascular access data. Having data in registries helps to inform future planning, guides practice, assists in future research and helps decide on resource allocation.19 In order to improve access utilisation in the haemodialysis population, one needs to evaluate the current practice.19
The aim of this study was therefore to examine the current and past use of AVFs, AVGs and CVCs in our unit, in light of national and international recommendations. This was done by performing an audit of all patients enrolled in the haemodialysis programme at our hospital. The objective was to identify any factors preventing this unit from achieving guideline targets and to propose changes that could be implemented to achieve a better haemodialysis access service.
Methods
Ethical clearance was obtained from the Faculty of Health Sciences Postgraduate Education, Training, Research and Ethics Unit of the Walter Sisulu University.
We performed an audit on the vascular access for chronic haemodialysis patients in Livingstone Tertiary Hospital in Port Elizabeth, South Africa. A retrospective folder review was done to identify demographic data and full vascular access history. The data were used to calculate the time from commencement of haemodialysis until the first attempt at the creation of a permanent vascular access. Each modality of haemodialysis access was then evaluated in the patient’s records. The date of insertion or creation of each modality was recorded. Where available, the complications associated with each were also recorded.
All patients enrolled in the haemodialysis programme at Livingstone Hospital on 1 June 2018, who had adequate records, were included in the study. Patients requiring temporary dialysis or awaiting transfer to peritoneal dialysis were excluded.
Results
Sixty-six patients formed the study sample, with age ranging from 21 to 67 years and a mean age of 44 years (95% CI: 42–46.8). Demographic details are shown in Table 1.
The majority of subjects [37 (56%)] were using a tunnelled CVC as their permanent vascular access, an autogenous AVF was used in 25 (38%) and an AVG in three (5%) patients. One patient was using a temporary CVC while awaiting a more definitive access modality (Table 2). Within the group that was using a CVC as permanent access, three subgroups were identified: those who had no AVF created or AVG inserted (12%), those with one previous failed AVF or AVG (21%), and those who had had more than one previous attempt at an AVF or AVG (23%).
Table 1. Demographic data of the 66 patients.
| Demographics | Values |
| Mean age, years (95% CI) | 44 (42–46.8) |
| Gender, n (%) | |
| Male | 35 (53) |
| Female | 31 (47) |
| Race, n (%) | |
| Black | 43 (65) |
| Coloured | 17 (23) |
| White | 6 (9) |
| Aetiology of renal failure, n (%) | |
| Hypertension | 50 (76) |
| Unknown | 6 (9) |
| Polycystic kidney disease | 3 (5) |
| Systemic lupus erythematosus | 3 (5) |
| Vesico–ureteric reflux | 2 (3) |
| Sepsis | 1 (2 |
| Glomerulonephritis | 1 (2) |
| Mean BMI, kg/m² (95% CI) | 24.4 (22.6–25.3) |
BMI, body mass index.
Central venous catheters were used in 95% of the studied patients as the initial modality. This included 38 patients (58%) who started with a temporary CVC and 25 (38%) who started with a tunnelled CVC. Only six (10%) patients had pre-emptive creation of permanent access, of which three were successfully used. The other three had a primary failure and had to have dialysis initiated using a CVC (Table 2).
Table 2. History of vascular access creation and complications.
| History | Number | Percentage of study populations |
| Current access | ||
| Tunnelled CVC | 37 | 56 |
| AVF | 25 | 38 |
| AVG | 3 | 5 |
| Temporary CVC | 1 | 2 |
| CVC group sub-analysis | ||
| Patients using a CVC at present | 37 | 56 |
| With no previous AVG or AVF | 8 | 12 |
| With 1 previous AVG or AVF | 14 | 21 |
| With > 1 previous AVG or AVF | 15 | 23 |
| Initial vascular access | ||
| CVC | 63 | 95 |
| Non-tunnelled CVC | 38 | 58 |
| Tunnelled CVC | 25 | 38 |
| Pre-emptive AVF | 3 | 5 |
| Number of AVF or AVG attempts | ||
| No previous AVF or AVG | 8 | 12 |
| 1 AVF or AVG | 29 | 44 |
| 2 AVF or AVG | 15 | 23 |
| 3 AVF or AVG | 13 | 20 |
| 4 AVF or AVG | 1 | 2 |
| Complications | ||
| Central venous stenosis or occlusion (of 66 patients) | 17 | 26 |
| Aneurysmal dilatation (of 101 AVFs) | 15 | 15 |
| Aneurysmal and still in use (of 15) | 9 | 60 |
| Aneurysmal and abandoned (of 15) | 6 | 40 |
| Dialysis access-associated steal síndrome | 0 | 0 |
CVC, central venous catheter; AVF, arteriovenous fistula; AVG, arteriovenous graft.
The timing from initiation of haemodialysis until the first attempt at AVF creation was also investigated (Fig. 1). In total, 101 AVFs were created in the study group. The number of access creation attempts and the complications experienced are shown in Table 2. There was no recorded episode of significant dialysis access-associated steal syndrome. The data were inadequate to calculate primary and secondary patency rates.
fig. 1.
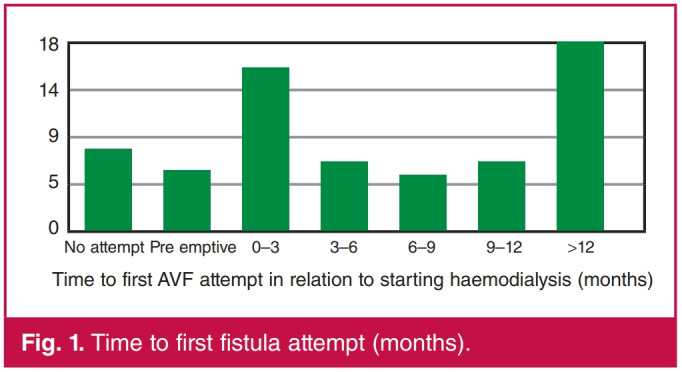
Time to first fistula attempt (months).
Discussion
Despite the young age of this population receiving haemodialysis in our unit, there was a high rate of CVC use and a very high rate of serious complications, such as clinically apparent central venous stenosis. The target for AVF use in any unit, as set by the Fistula First Breakthrough Initiative, is 65%. Several highincome countries are reporting AVF prevalence this high.7 Only 38% of our study population were using an AVF. The high rate of CVC use in this study is therefore not in keeping with guideline recommendations. However, on further investigation, it is clear that CVC use was not the primary strategy, since most of these patients had had prior AVFs that failed.
While only 12% of the entire group had never had, or was not then using an AVF, unfortunately 95% of the patients started dialysis using a CVC, with only 5% having a successful pre-emptive fistula. This reliance on CVCs increases the failure rate of AVFs created in the future as prior CVC use decreases the benefit of an AVF.11
While late presentation with advanced kidney disease is a common occurrence in our unit, necessitating the use of CVCs, long delays to creation of permanent access after starting dialysis prolongs exposure to the harmful effects of CVCs.11,20 Almost a third of patients in this study waited more than 12 months prior to the first AVF attempt. This most likely contributed to the high failure rate when an AVF was eventually created. Pre-emptive fistulae should ideally be fashioned three to six months before the first haemodialysis session to allow for maturation and re-intervention if necessary.5-7
These findings are similar to reports from sub-Saharan Africa as well as other low- and middle-income countries, where most patients will start dialysis on an emergency basis and cannot wait for a fistula to mature.16,21 This perpetuates the cycle, as higher rates of CVC use lead to poorer outcomes with AVFs, which lead to more CVC use. Except for the high primary failure rate, a lack of secondary intervention also decreases the long-term patency rates of the AVFs. Whenever failing fistulae are identified, rapid referral for intervention prior to a complete occlusion is required. Interventions done to maintain the fistula prior to complete occlusion are more likely to be successful.22 The access surgeon also requires available theatre time to be able to attempt salvage. When urgent secondary interventions are not available, the fistulae will simply be abandoned when they occlude.16
The documented complication rates may have been underestimated in this retrospective study as it relied on the adequacy of the patient records. Central venous stenosis or occlusion was recorded in a quarter of the patients. This may even be an underestimation, since patients are not routinely screened for evidence of central venous obstruction and only clinically apparent central venous obstruction was recorded. The damage caused by long-term CVC use leads to central venous stenosis and can compromise future access options.23
Recommendations for improving current practice
Early detection of CKD and timely referral: many patients present late with end-stage kidney disease. Ongoing education of healthcare providers is needed to promote early referral. Early detection of CKD may avoid the need for urgent dialysis and therefore CVC use, allowing time for pre-emptive access creation.24
Dedicated vascular access clinic: a specialised multidisciplinary clinic should be formed that deals primarily with new and problematic vascular access cases.18 This multidisciplinary team should include a vascular access surgeon, a nephrologist, dialysis nursing staff and supporting staff. All new referrals can be seen and access planning started prior to the first dialysis session. Ultrasound evaluation can be done at the initial visit to map out potential access sites and look for problematic areas such as prior vein injury by cannulation. When there are concerns regarding early AVF failure, intervention can then be planned and the patient prioritised for surgical revision from this clinic. In this format there will be open communication between the different members of the haemodialysis team. It will also allow time for patient education in a neutral environment with all the different team members available.
Availability of a dedicated vascular access theatre list: without access to theatre it would not be possible to run an effective vascular access service. The best way to optimise the timing to AVF creation and deal with failing fistulae or complications would be to allocate a dedicated vascular access theatre list. This list should ideally be in a hybrid theatre or a theatre with fluoroscopy available so that both open surgical and endovascular interventions can be performed as needed. The haemodialysis patients can then be prioritised and would not need to compete for theatre time with all the other emergency and elective surgical patients.
A dedicated access co-ordinator: it would be valuable to appoint a dedicated vascular access co-ordinator. This should be a trained nurse experienced in haemodialysis and vascular surgery. Ideally one of the experienced nurses currently in the unit could fulfil this role. The co-ordinator will be the link between the patient, dialysis staff, nephrologist and access surgeon. This strategy has been shown to be very effective in improving haemodialysis outcomes.18
Prospective data collection and interval evaluation: having a registry of vascular access data can help to plan resource allocation, guide the design of future clinical trials and monitor the local vascular access practice. There is currently no prospective registry for vascular access in South Africa, although the South African Renal Registry does collect data on haemodialysis across South Africa.25 The newly established registry of the African Association of Nephrology will collect valuable data on CKD management across Africa but lacks data collection on vascular access.26 Adding vascular access data to these registries would be beneficial to the African dialysis population.
Conclusion
It is a great privilege to be able to offer chronic haemodialysis to our patients. To make the most use of this service we need to optimise their vascular access. Our current practice falls short of local and international guidelines in terms of AVF and CVC use. The overwhelming majority of patients start dialysis with a CVC rather than a recommended pre-emptive AVF and there are significant delays prior to the first AVF creation. This translates to a longer time using a CVC and increased complications as well as limiting future access options. Recommendations to improve the service would be to create a multidisciplinary vascular access clinic, establish a dedicated vascular access theatre list and assign an access co-ordinator. Ongoing education of healthcare practitioners on the earlier identification and referral of kidney disease will facilitate pre-emptive creation of vascular access.24 Finally, there is also a need for a South African vascular access registry to identify the local practices of each haemodialysis unit.
Contributor Information
Ian R Grant, Email: ianroygrant@gmail.com.
Robert J Freercks, Division Nephrology, Department of Medicine, Livingstone Tertiary Hospital, Port Elizabeth; Division Nephrology and Hypertension, Department of Medicine, University of Cape Town, Cape Town, South Africa.
References
- 1.Naicker S. End-stage renal disease in sub-Saharan Africa. Kidney Int. 2013;3(Suppl):161–163. doi: 10.1046/j.1523-1755.63.s83.25.x. [DOI] [PubMed] [Google Scholar]
- 2.Barsoum RS, Khalil SS, Arogundade FA. Fifty years of dialysis in Africa: Challenges and progress. Am J Kidney Dis. 2015;65(3):502–512. doi: 10.1053/j.ajkd.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Ranisinghe P, Perera YS, Makarim MFM, Wijesinghe A, Wanigasuriya K. The costs in provision of haemodialysis in a developing country: A multi-centered study. BMC Nephrol. 2011;12(42):1–7. doi: 10.1186/1471-2369-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashuntantang G, Osafa C, Olawu WA, Arongundale F, Niang A, Porter J. et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet. 2017;5:408–417. doi: 10.1016/S2214-109X(17)30057-8. [DOI] [PubMed] [Google Scholar]
- 5.Paget G, Naicker S. Guideline for the optimal care of patients on chronic dialysis in South Africa. South African Renal Society, 2015 [Google Scholar]
- 6.Schmidli J, Widmer MK, Basile C, de Donato G, Gallieni M, Gibbons CP. et al. 2018 Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55:757–818. doi: 10.1016/j.ejvs.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation. KDOQI 2006 vascular access guidelines. Am J Kidney Dis. 2006;48:177–322. [Google Scholar]
- 8.Donati G, Cianciolo G, Mauro R, Rucci P, Scrivo A, Marchetti A. et al. PTFE grafts versus tunneled cuffed catheters for hemodialysis: which is the second choice when arteriovenous fistula is not feasible? Artif Organs. 2014;39(2):134–141. doi: 10.1111/aor.12353. [DOI] [PubMed] [Google Scholar]
- 9.Vesely T, DaVanzo W, Behrend T, Dwyer A, Aruny J. Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. J Vasc Surg. 2016;64(5):1400–1410. doi: 10.1016/j.jvs.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Ramon RT. Permanent arteriovenous fistula or catheter dialysis for heart failure patients. J Vasc Access. 2016;17(1):23–29. doi: 10.5301/jva.5000511. [DOI] [PubMed] [Google Scholar]
- 11.Yuo TH, Chaer RA, Dillavou ED, Leers SA, Makaroum MS. Patients started on hemodialysis with tunnelled dialysis catheter have similar survival after arteriovenous fistula and arteriovenous graft creation. J Vasc Surg. 2015;62(6):1590–1597. doi: 10.1016/j.jvs.2015.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fokou M, Teyang A, Ashuntantang G, Kaze F, Eyenga VC, Chicom Mefire A. et al. Complications of arteriovenous fistula for hemodialysis: an 8-year study. Ann Vasc Surg. 2012;26(5):680–684. doi: 10.1016/j.avsg.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Alashek WA, McIntyre CW, Taal MW. Vascular access in patients receiving hemodialysis in Libya. J Vasc Access. 2012;13(4):468–474. doi: 10.5301/jva.5000089. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed GM, Mansour MO, Elfatih M, Khalid KE, Ahmed Mel I. Outcomes of arteriovenous fistula for hemodialysis in Sudanese patients: single-center experience. Saudi J Kidney Dis Transpl. 2012;23(1):152–157. [PubMed] [Google Scholar]
- 15.Kolb I, Twangirumugabe T, Uyisabye I, Muhizi J, Braun-Parvez L, Richter S. Emergency vascular access conversion to native arteriovenous fistula: a prospective study of 37 hemodialysis patients in Rwanda. Nephrol Ther. 2014;10(6):457–462. doi: 10.1016/j.nephro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Alhassan SU, Adamu B, Abdu A, Aji SA. Outcome and complications of permanent hemodialysis vascular access in Nigerians: A single centre experience. Ann Afr Med. 2013;12(2):127–130. doi: 10.4103/1596-3519.112410. [DOI] [PubMed] [Google Scholar]
- 17.Ilhan G, Esi E, Bozok S, Yurekli I, Ozpak B, Ozelci B. et al. The clinical utility of vascular mapping with Doppler ultrasound prior to arteriovenous fistula construction for hemodialysis access. J Vasc Access. 2013;14(1):83–88. doi: 10.5301/jva.5000097. [DOI] [PubMed] [Google Scholar]
- 18.Lancashire JF, Steele M, Swinbank A, Du Toit D, Jackson MJ. A 10-year review of a vascular access service for patients receiving hemodialysis: analysis of procedural modifications and service innovations. J Assoc Vasc Access. 2016;21(2):93–102. [Google Scholar]
- 19.Davids RM, Caskey FJ, Young T, Singh GKB. Strengthening renal registries and ESKD research in Africa. Sem Nephrol. 2017;37(3):211–223. doi: 10.1016/j.semnephrol.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Whittier WL. Surveillance of hemodialysis vascular access. Sem Int Rad. 2009;26(2):130–138. doi: 10.1055/s-0029-1222457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemachandar R. Analysis of vascular access in haemodialysis patients – single center experience. J Clin Diag Res. 2015;9(10):1–4. doi: 10.7860/JCDR/2015/13342.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allon M (ed 2017). Monitoring and surveillance of hemodialysis arteriovenous grafts to prevent thrombosis. Up to date, 2017. Available at: https://www.uptodate.com/contents/monitoring-and-surveillance-ofhaemodialysis- arteriovenous-grafts-to-prevent-thrombosis [Accessed 10 March 2019]. [Google Scholar]
- 23.Jakimowicz T, Galazka Z, Grochowiecki T, Nazarewski J, Szmidt J. Vascular access for haemodialysis in patients with central vein thrombosis. Eur J Vasc Endovasc Surg. 2011;42:842–849. doi: 10.1016/j.ejvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Karkar A. Caring for patients with CRF: rewards and benefits. Int J Nephrol. 2011;2011:1–6. doi: 10.4061/2011/639840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davids MR, Jardine T, Marais N, Jacobs JC. South African Renal Registry annual report 2016. Afr J Neph. 2018;21(1):61–72. [Google Scholar]
- 26.Davids R, Eastwood JB, Selwood NH, Arogundade A, Ashuntantang G, Gharbi MB. A renal registry for Africa: First steps. Clin Kidney J. 2016;9(1):162–167. doi: 10.1093/ckj/sfv122. [DOI] [PMC free article] [PubMed] [Google Scholar]


