Abstract
Treating advanced lung cancer in patients with pulmonary fibrosis can be challenging due to increased risks of drug‐induced and radiation‐induced pneumonitis. We present the case of a 78‐year‐old female with stage IIIB lung adenocarcinoma and idiopathic pulmonary fibrosis (IPF). Following partial response to platinum‐based chemotherapy, she was started on maintenance pemetrexed, but this was stopped due to bone marrow suppression and kidney injury. She received no further chemotherapy. Nintedanib was subsequently commenced for treatment of IPF. Remarkably, progress imaging at 2 years showed further regression of her lung adenocarcinoma, with stability of IPF. Nintedanib is a multi‐target tyrosine kinase inhibitor with anti‐cancer effects due to inhibition of angiogenesis. Nintedanib with chemotherapy has shown improvements in survival in non‐small cell lung cancer (NSCLC). This case report and literature review discuss the effectiveness of nintedanib in treating patients with concurrent IPF and NSCLC, in particular for patients poorly tolerant of conventional chemotherapy.
Keywords: idiopathic pulmonary fibrosis, interstitial lung disease, lung cancer, nintedanib, non‐small cell lung cancer
This case report is of a 78‐year‐old female with concurrent non‐small cell lung cancer (NSCLC, stage IIIB) and idiopathic pulmonary fibrosis (IPF). After no longer able to continue conventional chemotherapy, the patient was commenced on nintedanib to treat IPF and interestingly had continued radiological regression of her NSCLC over a 2‐year period with stability of IPF. This case report and literature review discuss the effectiveness of nintedanib in treating patients with concurrent IPF and NSCLC, in particular for patients poorly tolerant of conventional chemotherapy.
INTRODUCTION
Nintedanib is an oral tyrosine kinase inhibitor against multiple targets including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet‐derived growth factor (PDGF). 1 It is approved and funded for the treatment of idiopathic pulmonary fibrosis (IPF) under the Pharmaceutical Benefit Scheme (PBS) in Australia. Nintedanib in combination with docetaxel is also used in Europe as a second‐line treatment for recurrent or metastatic non‐small cell lung cancer (NSCLC), with improvement in progression‐free and overall survival. 1 However, limited data exist regarding nintedanib as a single agent against NSCLC, or in the context of concurrent IPF. This is important considering that radiological features of IPF are present in up to 17% of patients with lung cancer. 1
CASE REPORT
We present a 78‐year‐old female referred for an incidental 30 mm spiculated lung nodule in the left lower lobe, which was present on her computed tomography (CT) abdomen performed for abdominal pain. A subsequent CT chest confirmed the nodule and identified concurrent bilateral basal intralobular septal thickening consistent with an interstitial lung disease (ILD). Her background history included right total nephrectomy for renal cell carcinoma in 2012 with no adjuvant treatment. She had hypertension, hypercholesterolaemia and vitamin D deficiency. She was an ex‐smoker of 25 pack‐years, but otherwise had no occupational or environmental exposure to organic and inorganic dust. Physical assessment showed no clubbing or palpable lymphadenopathy. Her oxygen saturation was 97% whilst breathing room air. Chest auscultation revealed intermittent inspiratory crackles. Lung function testing showed mild restriction with reduced total lung capacity at 76% of predicted, forced vital capacity (FVC) at 94% of predicted, forced expiratory volume in 1 s to FVC ratio of 0.79 and moderately reduced diffusing capacity for carbon monoxide at 50% of predicted.
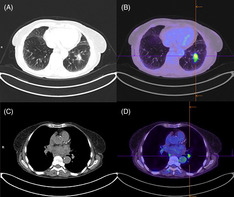
She underwent a positron emission tomography scan which showed moderate avidity of the left lower lobe nodule, as well as mediastinal and left hilar lymphadenopathy (Figure 1). An endobronchial ultrasound transbronchial nodal aspirate confirmed involvement of lymph node station 11L but also contralateral station 11R, and she was diagnosed with stage IIIB lung adenocarcinoma. Immunohistochemistry was positive for programmed cell death ligand‐1 (PDL‐1) at 100%, but negative for other mutations including epidermal growth factor receptor.
FIGURE 1.
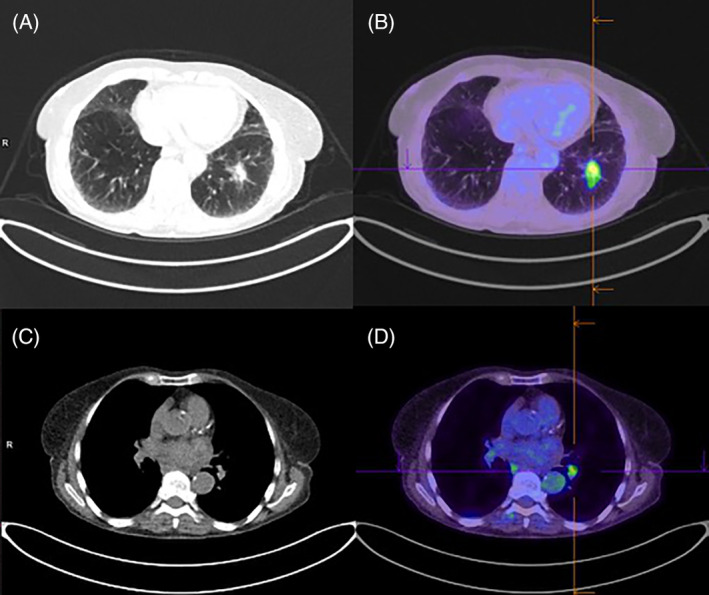
Computed tomography/positron emission tomography (CT/PET) scan in July 2018 showing left lower lobe nodule (A) on CT, with moderate fluorodeoxyglucose (FDG) avidity (maximum standardized uptake value [SUVmax] 5.7) on PET (B), and left hilar lymphadenopathy (C) with moderate FDG avidity (SUVmax 4.3) (D)
The standard treatment for stage IIIB NSCLC then was concurrent chemotherapy and radiotherapy, which was contraindicated due to risk of worsening pulmonary fibrosis from radiotherapy. There was furthermore significant concern regarding the use of anti‐PDL‐1 immunotherapy due to the risk of drug‐induced pneumonitis. She was therefore treated with palliative chemotherapy with carboplatin/gemcitabine in September 2018 followed by maintenance pemetrexed in January 2019 with palliative intent. However, whilst on pemetrexed, she developed severe exertional dyspnoea and a repeat CT chest revealed new bilateral ground‐glass opacities concerning for possible chemotherapy‐induced pneumonitis. She was commenced on a course of prednisone with gradual resolution of the ground‐glass changes. Around the same time, she developed bone marrow suppression and worsening renal function likely due to pemetrexed, and therefore she was not deemed for further chemotherapy for her lung cancer. By March 2019, there was partial response with the lesion measuring 17 × 13 mm (Figure 2A).
FIGURE 2.
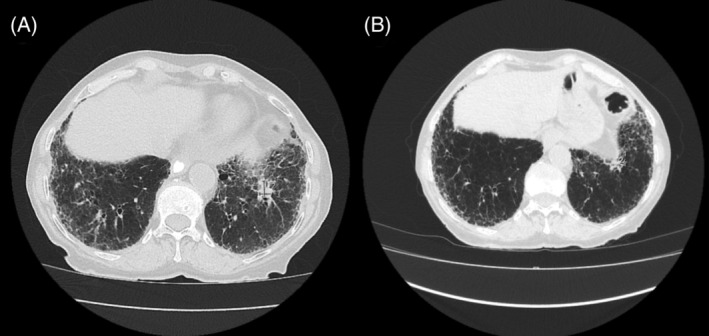
Computed tomography chest scans showing left lower lobe nodule pre (A, March 2019) and post (B, July 2021) nintedanib monotherapy
Meanwhile, her underlying ILD had progressed with worsening of bilateral subpleural reticulation. She had a negative autoimmune/myositis panel. Following discussion in our ILD multidisciplinary meeting, the patient was commenced on nintedanib 150 mg BD in June 2019 without a lung biopsy. Besides diarrhoea, she tolerated the nintedanib well. Progress CT imaging showed improving pulmonary fibrosis but, interestingly, there was further progressive reduction in the size of the lung cancer, measuring 7 × 4 mm in July 2021 (Figure 2B).
DISCUSSION
We present a patient who was treated with nintedanib for IPF, but also had radiological regression of her NSCLC over a 2‐year period. Although nintedanib is recommended as an anti‐fibrotic agent for IPF, it can exhibit anti‐cancer effects due to the inhibition of angiogenesis via blocking VEGF, PDGF and FGF. 1
In a multi‐centre randomized placebo‐controlled trial of 1314 patients, Paliogiannis et al. demonstrated that the addition of nintedanib to docetaxel chemotherapy resulted in improved progression‐free survival for NSCLC (3.4 vs. 2.7 months, p = 0.002) and improved overall survival for adenocarcinoma (12.6 vs. 10.3 months, p = 0.036). 1 Based on these data, nintedanib with docetaxel is offered as second‐line therapy for recurrent adenocarcinoma in European and UK guidelines. Furthermore, a phase II trial showed nintedanib with pemetrexed achieved longer progression‐free survival compared with pemetrexed alone. 1
However, there are limited data exploring the effectiveness of nintedanib as a single agent against NSCLC in patients with co‐morbid IPF. Essentially, we found two Japanese case reports. The first case details a 69‐year‐old with stage IIIA NSCLC and IPF, who was commenced on nintedanib for progressive fibrosis and had subsequent radiological regression of the lung mass after 1 month. 2 The second case reports a 76‐year‐old, who achieved stability in both a 13.5 × 11.7 mm lung nodule and concurrent IPF whilst taking nintedanib monotherapy for 9 months. Unfortunately, nintedanib was ceased due to severe gastrointestinal side effects. Within the subsequent 4 months, the lung nodule enlarged to 20.8 × 22.0 mm and was revealed to be squamous cell carcinoma on surgical resection. 3
Whilst concurrent IPF and NSCLC have low prevalence, the presence of IPF is associated with a five‐fold increased risk of lung cancer, 4 and the development of lung cancer reduces median survival in IPF patients by 25.2 months. 5 Further research into the treatment of concurrent IPF and NSCLC is warranted, as this case has demonstrated an effectiveness of nintedanib as a single agent in controlling both disease processes.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Shakti Dabholkar wrote the initial manuscript including the discussion. Brian Chuong obtained informed consent from the patient and provided expert opinion in respiratory medicine. Bo Gao provided expert opinion in medical oncology. All authors reviewed and contributed to the final manuscript.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Dabholkar S, Gao B, Chuong B. Nintedanib—A case of treating concurrent idiopathic pulmonary fibrosis and non‐small cell lung cancer. Respirology Case Reports. 2022;10:e0902. 10.1002/rcr2.902
Associate Editor: Kazuhisa Takahashi
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Paliogiannis P, Fois SS, Fois AG, Cossu A, Palmieri G, Pintus G. Repurposing anticancer drugs for the treatment of idiopathic pulmonary fibrosis and antifibrotic drugs for the treatment of cancer: state of the art. Curr Med Chem. 2021;28:2234–47. 10.2174/0929867327999200730173748 [DOI] [PubMed] [Google Scholar]
- 2. Shiratori T, Tanaka H, Tabe C, Tsuchiya J, Ishioka Y, Itoga M, et al. Effect of nintedanib on non‐small cell lung cancer in a patient with idiopathic pulmonary fibrosis: a case report and literature review. Thorac Cancer. 2020;11:1720–3. 10.1111/1759-7714.13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fukunaga K, Yokoe S, Kawashima S, Uchida Y, Nakagawa H, Nakano Y. Nintedanib prevented fibrosis progression and lung cancer growth in idiopathic pulmonary fibrosis. Respirol Case Rep. 2018;6(8):e00363. 10.1002/rcr2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med. 2007;101(12):2534–40. [DOI] [PubMed] [Google Scholar]
- 5. Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015;147(1):157–64. 10.1378/chest.14-0359 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


