ABSTRACT
The etiology of type 2 diabetes is multifactorial, in which environmental and genetic factors are involved to varying degrees. This suggests that its pathophysiology might vary depending on the individuals. Knowledge of the differences is critical, because these differences are directly linked to the care and treatment of the patients. Recent studies have attempted to carry out subclassifications of type 2 diabetes based on clinical and genetic differences. However, there is no pathological evidence to support these subclassifications. The pathophysiology of type 2 diabetes is generally divided into insulin resistance in peripheral tissues and pancreatic islet dysfunction. Among them, islet dysfunction causes a deficit in insulin secretion from β‐cells. In particular, a deficit in insulin secretion is ascribed to a combination of disruption of the insulin secretory machinery and a decrease in β‐cell volume in type 2 diabetes. Recent research has suggested that transdifferentiation and dedifferentiation are involved in the decrease in β‐cell volume, and that it might change dynamically depending on the glucose metabolic state. However, it is possible that the numbers of islet cells are decreased in type 2 diabetes. In particular, the loss of endocrine cells due to islet amyloid deposits is an important pathological change in type 2 diabetes in humans. These results show that pathological changes of the islets can be different in each individuals with type 2 diabetes and reflect each pathophysiology, which is useful in establishing further subclassifications and developing tailor‐made therapies for type 2 diabetes.
Keywords: Amyloid, Islet pathology, Type 2 diabetes
Pathological findings of the islets can reflect the pathophysiologies of type 2 diabetes. Detailed pathological analysis enable to subclassify type 2 diabetes.
DIVERSE PATHOGENESIS OF TYPE 2 DIABETES
The current epidemic of type 2 diabetes is a serious concern in modern society worldwide 1 . Effective prevention or cure of this disorder has yet to be established. One of the reasons for this difficulty might be the diversity of the clinical and pathophysiological background. Such heterogeneity might largely be influenced by different ethnicities or life customs. Peripheral insulin resistance and deficient insulin secretion are salient features of type 2 diabetes 2 . Insulin resistance is associated with a high body mass index (BMI; average 27.5) in white type 2 diabetes patients, but not in Japanese type 2 diabetes patients (average BMI 23.4) 3 , 4 , 5 , 6 . Several studies disclosed that impaired insulin secretion rather than insulin resistance was more closely associated with the development of diabetes in Japanese individuals 3 , 4 . Differences in islet pathology between white patients and Japanese patients with or without type 2 diabetes have also been recently presented 5 , 6 , 7 , 8 , 9 , 10 . For example, the prevalence of islet amyloid deposition is >80% in white type 2 diabetes patients, whereas it is just 30% in Japanese type 2 diabetes patients 9 , 10 . An obesity‐associated increase in β‐cell volume density is well documented in white individuals 8 , whereas it is not the case in Japanese individuals 5 , 6 . Thus, the diversity of pathological changes in the islets might reflect the background pathophysiology of each individual.
SUBCLASSIFICATION OF TYPE 2 DIABETES
Recently, many attempts have been made to subclassify type 2 diabetes. Swedish investigators classified type 2 diabetes into five subgroups of autoimmune‐based type, insulin deficient type, aging‐related type, obesity‐related type and insulin‐resistant type by measures of glutamic acid decarboxylase antibody, age, BMI, glycated hemoglobin, homeostasis model assessment of β‐cell function and homeostasis model assessment of insulin resistance 11 . Among these, they found that a group of type 2 diabetes patients with severe insulin resistance developed a high prevalence of chronic kidney disease and cardiovascular events with poor prognosis 11 . The German group also classified type 2 diabetes into five subgroups of mild age‐related diabetes, mild obesity‐related diabetes, severe autoimmune diabetes, severe insulin‐resistant diabetes and severe insulin‐deficient diabetes based on age, BMI, glycemia, homoeostasis model estimates and islet autoantibodies 12 . Interestingly, the prevalence of diabetic complications, particularly diabetic neuropathy, was the highest in the severe insulin‐resistant diabetes group among all groups. The underlying change in pathology in the islets for the aforementioned subgroups of type 2 diabetes remains, however, to be addressed. It is important to clarify the difference in pathologies in those classifications, because it will determine the therapy for each patient. In the present review, we discuss the diversity of islet pathology relating to clinical characteristics, which can promote further subclassification and tailor‐made therapies for type 2 diabetes.
ALTERATION OF ISLET PATHOLOGY IN TYPE 2 DIABETES
A main pathogenesis of type 2 diabetes is abnormal hormone secretion in response to glucose from the islet, such as the deficiency of insulin secretion in β‐cells and no suppression of hyperglycemic glucagon hypersecretion in α‐cells 13 , 14 . Pathologically, it is known that the β‐cell volume decreases, the α/β‐cell ratio increases and the α‐cell volume increases 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 (Figure 1). Decreased β‐cell volume is directly linked to less insulin secretion, whereas the increase in α‐cell volume might interfere with the reduction of glucose levels after meal ingestion. In contrast, it is known that alteration of endocrine cell composition in the islets also changes endocrine cell cross‐talk in the islets and affects hormone secretion 24 , 25 , 26 , 27 . β‐cells secrete humoral factors, such as insulin, gamma aminobutyric acid, zinc and glutamate, which can inhibit glucagon secretion from cells 24 , 25 , 26 , 27 . Thus, the alteration of endocrine cell composition in the islets is directly linked to the function of the islets.
Figure 1.
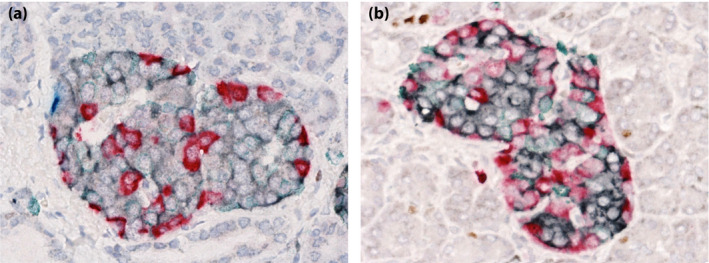
Morphological changes in the islets of type 2 diabetes patients. Quadruplicate immunostained sections (red, glucagon; black, insulin; green, somatostatin; blue, PP; brown, Ki67) are shown. (a) Individuals without diabetes. (b) Individuals with diabetes). The occupancy of insulin‐positive cells is decreased and that of glucagon‐positive cells is increased in type 2 diabetes patients compared with individuals without diabetes.
Several mechanisms for β‐cell reduction have been proposed, including β‐cell death and transdifferentiation 17 , 22 , 28 , 29 , 30 (Figure 2). In type 2 diabetes, islet neogenesis can occur to compensate for β‐cell reduction, and β‐cell proliferation is not influenced 20 , 21 . The decrease in β‐cell volume can depend on the blood glucose status 18 . The frequency of β‐cell apoptosis is extremely low or difficult to find. In addition, the islet volume itself is not significantly reduced in type 2 diabetes 20 , 21 , 22 , 23 . These findings might suggest that transdifferentiation and dedifferentiation could be major players in β‐cell loss rather than β‐cell death in the short term. Nevertheless, because type 2 diabetes is a multifactorial disease, islet pathological changes might also vary with different involvement of pathophysiological factors among patients with type 2 diabetes.
Figure 2.
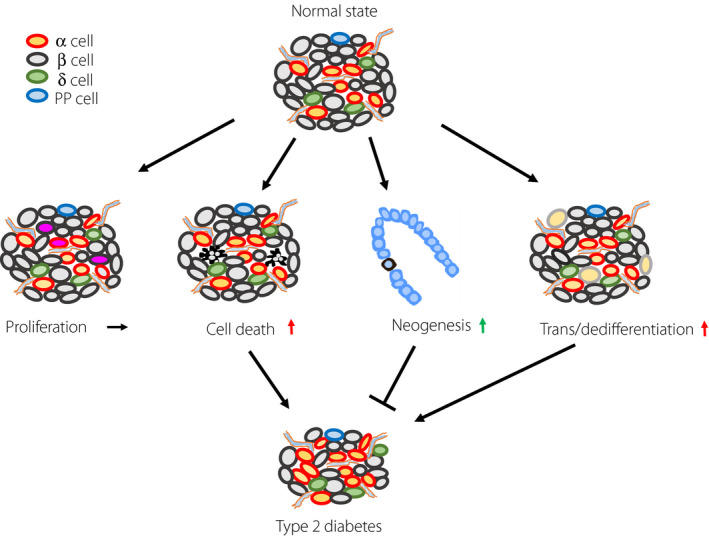
Mechanism of endocrine cell kinetics for the reduction of β‐cells in type 2 diabetes patients. Pancreatic islets have a cell maintenance mechanism, which regulates a proper balance of endocrine cell proliferation, cell death, neogenesis and trans/dedifferentiation. In type 2 diabetes patients, this balance is disturbed by harmful factors, such as oxidative stress, endoplasmic reticulum stress and deficiency of autophagy. Consequently, the proliferative capacity of endocrine cells does not change, whereas cell death and trans/dedifferentiation increase. Islet neogenesis is thought to increase in a compensatory manner.
ISLET AMYLOID DEPOSITS IN TYPE 2 DIABETES
Amyloid deposits are characteristic pathological changes in type 2 diabetes 10 , 21 , 29 , 31 (Figure 3). Amyloid in type 2 diabetes consists of islet amyloid polypeptide. Islet amyloid polypeptide, also known as amylin, is a 37‐amino acid peptide hormone. Amylin is the second most abundant peptide secreted by β‐cells 32 . Amylin is released in response to nutrients, including glucose, lipids or amino acids (arginine) 33 , 34 . Pro‐amylin is processed by cleavage at basic residue pairs at both its amino and carboxyl termini with prohormone convertase 1/3 and prohormone convertase 2, and its C‐terminus is amidated by carboxypeptidase E and peptidylglycine alpha‐amidating monooxygenase 35 . Mature amylin is secreted along with insulin, which is stored in the same granules 36 . The molecular mechanisms that result in refolding of the peptide to convert it from a normally soluble monomer into insoluble fibrils have not been identified. Amyloid deposition increases the frequency of apoptotic β‐cells in white individuals 29 , 30 , but can increase deoxyribonucleic acid fragmentation of β‐cells in Japanese type 2 diabetes patients 11 . Similarly, amylin aggregates drive inflammation elicited by pro‐inflammatory macrophages, resulting in β‐cell dysfunction 11 , 37 . Interestingly, amyloid deposition suppresses the increase in α‐cells and decreases chromogranin‐A endocrine cells 10 , 20 , 22 (Figure 3). These results suggest that aggregation of this peptide results in the formation of islet amyloid deposits, which can be toxic to not only β‐cells, but also other kinds of endocrine cells in type 2 diabetes patients. The individuals with amyloid deposition in the islets present with specific etiologies and would have different pathophysiology from the individuals without amyloid deposition (Figure 3). Thus, type 2 diabetes patients can be classified according to amyloid deposition in the islets.
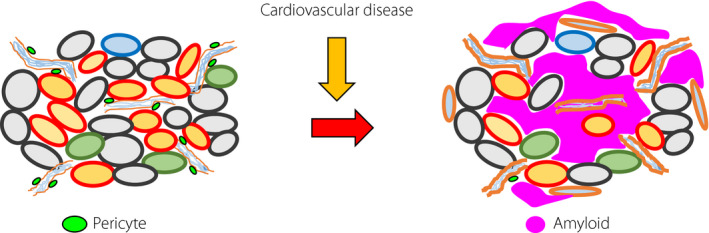
Figure 3.

Promoting factors for amyloid deposition in the islets. Complications involving type 2 diabetes, aging, obesity/insulin resistance, cardiovascular disease, chronic pancreatitis and renal failure can promote amyloid formation in the islets (red arrow). This deposition of amyloid reduces the cell volume of both β‐ and α‐cells in type 2 diabetes patients. Concurrently, inflammatory reactions are evoked in islets.
ETHNICITY
As aforementioned, islet pathology can be different between white patients and Japanese patients. In response to obesity, islets in white patients can show hyperplasia to compensate for the demand for insulin from peripheral tissues 8 . In contrast, the morphology of islets in Japanese individuals is less influenced by obesity 5 , 6 . Furthermore, the prevalence of amyloid deposits in the islets of white individuals is more >80%, whereas that in Japanese individuals is approximately just 30–40% 9 , 10 , 21 , 29 . Butler et al. showed that islet amyloid deposition was not increased in type 2 diabetes patients with a BMI of 27 kg/m2 compared with lean patients with a BMI of 25 kg/m2, nor was it increased in obese individuals with impaired glucose tolerance compared with either obese or lean individuals without diabetes in a recent autopsy study of 124 white individuals 17 . In contrast, as shown in a Chinese article, BMI is correlated with amyloid occupancy in Japanese individuals 10 . These results suggest that amyloid deposition is dependent on ethnic differences. To date, genetic differences to explain the morphological changes in islets have not been identified among different ethnicities.
OBESITY AND INSULIN RESISTANCE
Islet amyloid deposition might be a result of obesity‐associated insulin resistance 10 , 18 , 38 . Heterozygous transgenic mice expressing human amylin in pancreatic β‐cells show islet amyloid deposition when crossed with an obese, insulin‐resistant diabetic strain 39 . As is the case with insulin, patients with obesity have high basal and stimulated levels of plasma amylin 40 , 41 . As mentioned before, insulin secretion rather than insulin resistance was more closely associated with the development of diabetes in Japanese individuals 3 , 4 . In contrast, there might be individuals who maintain sufficient insulin secretion among Japanese individuals with obesity, particularly in the initial stage of the disease, where hyperinsulin and amylin secretion can occur to compensate for peripheral insulin resistance.
HYPERAMYLINEMIA AND CARDIOVASCULAR DISEASE
Elevation of systolic and diastolic blood pressure, and the presence of hypertension are risk factors for amyloid deposition in Chinese individuals 18 . These findings can be interpreted either as a cause or effect of hyperamylinemia. Hyperamylinemia promotes amylin deposition in the heart, causing alterations in cardiac myocyte structure and function 42 . Amylin deposition negatively affects cardiac myocytes by inducing sarcolemmal injury, generating reactive aldehydes, forming amylin‐based adducts with reactive aldehydes and increasing the synthesis of interleukin‐1β independent of hyperglycemia 43 . Amylin deposition in the heart failure of non‐human primates activates hypoxia‐inducible factor‐1α and 6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 3 signaling 44 . Verma et al. 45 reports that hypersecretion of human amylin is associated with amylin deposition in the microvasculature of the kidney and red blood cells, leading to impaired red blood cell–capillary interactions and activation of hypoxia signaling pathways, and resulting in the disruption of microvasculature. Thus, hyperamylinemia directly influences the function and cell viability of cardiovascular systems. Hyperamylinemia can also be associated with single‐nucleotide polymorphisms in the amylin gene. The −132 G/A mutation is located within an activator domain of the amylin gene promoter 46 . The prevalence of the mutation was more frequently observed in diabetes patients than in the control population with high serum amylin levels (10.1 vs 0.9%). Interestingly, hypertension was higher in a population of diabetes patients carrying the mutation than in diabetes patients who are non‐carriers (74 vs 57%; P < 0.05). These results suggest that hyperamylinemia induces cardiovascular disease, including hypertension and heart failure.
ISLET AMYLOID DEPOSITION AND MACRO‐ AND MICROANGIOPATHIES OF DIABETES
Conversely, as insulin resistance is a risk factor for both macroangiopathy and amyloid deposition in the islets, there is a possibility that the presence of circulatory disease itself is a risk factor for amyloid deposition in the islets. Recent studies have shown that macroangiopathy is a risk factor for microangiopathy in type 2 diabetes 47 , 48 . Thickened basement membrane (BM) and loss of pericytes are hallmarks of microangiopathy in diabetes, and long‐standing metabolic aberrations, such as increased polyol pathways and glycation, might underlie its development 49 , 50 . Generally, thickened BM obstructs drainage of amyloid‐related protein, resulting in amyloid formation 51 , 52 . Nevertheless, to date, the correlation between diabetic microangiopathy and amyloid deposition has not been fully clarified in type 2 diabetes. As the pancreatic islet is a highly vascularized endocrine micro‐organ 23 , 53 , it is plausible that microangiopathy of the islet vasculature contributes to amyloid deposition in type 2 diabetes patients.
The islets of ob/ob mice, which is a model of an obese type 2 diabetes similar to white type 2 diabetes patients, show dilation of islet capillaries and a reduction in pericyte lining, which might be an adaptive response to a high demand for insulin secretion due to insulin resistance 54 , 55 . In Goto‐Kakizaki rats, a lean type 2 diabetes model, hypercholesterolemia interacts with chronic hyperglycemia to induce islet microangiopathy, which can be associated with a reduction in the β‐cell mass 56 . In white patients with type 2 diabetes, islet microvessels showed thickened walls similar to those of microangiopathy observed in other tissues 51 . These alterations of islet microvasculature might contribute to amyloid deposition in the islets of human type 2 diabetes.
EXAMINATION OF CARDIOVASCULAR DISEASE AND AMYLOID DEPOSITION USING AUTOPSY PANCREAS SAMPLES
To explore the hypothesis described earlier, a study was carried out on the autopsy pancreas samples of Japanese type 2 diabetes patients with complications of acute myocardial infarction (AMI) as a hallmark of cardiovascular disease 23 . Clinically, systolic blood pressure, total cholesterol and prevalence of renal failure were more elevated in type 2 diabetes patients with AMI than in those without AMI. Furthermore, in pathological evaluations, amyloid deposits, BM thickening, loss of pericytes and increase in vascular density in the islets were significantly observed in type 2 diabetes patients with AMI (Figure 4). These results suggest that type 2 diabetes patients with AMI might be closely related to the insulin resistance group classified by Ahlqvist et al. 11 (Table 1). Namely, amyloid deposits might reflect islet pathological features of the insulin‐resistant group. In the evaluation of the microvessels in the islets of type 2 diabetes patients with AMI, the thickening of BM and the loss of pericytes were remarkable. These changes are similar to the microangiopathy of diabetes. It is conceivable that microangiopathy‐like changes in pancreatic islets might be exerted due to macroangiopathy complications. Such vascular changes can reduce vascular permeability for amylin and promote amyloid deposition.
Figure 4.
Pathological changes in islets in type 2 diabetes patients with acute myocardial infarction. Type 2 diabetes complicated with acute myocardial infarction shows amyloid deposition, thickening of the basement membrane and loss of pericyte coverage of microvessels in the islets. These changes are similar to those in microvascular complications of diabetes.
Table 1.
Similarity of acute myocardial infarction complicated type 2 diabetes to insulin resistance type
| Insulin resistance type | Type 2 diabetes + AMI | |
|---|---|---|
| High prevalence of cardiovascular events | + | + |
| High prevalence of renal failure | + | + |
| Islet amyloid deposition | Possibly | + |
| Insulin resistance | + | Possibly |
AMI, acute myocardial infarction.
RENAL FAILURE AND AMYLOID DEPOSITION
Systemic amyloidosis can occur when there is a high serum concentration of protein precursors, as seen in long‐term hemodialysis patients with increased levels of β‐2‐microglobulin. Under normal circumstances, amylin is released into the circulation and is excreted through the kidney 40 , 57 . Similarly, the presence of renal insufficiency is known as a risk factor for amyloid deposition in the islets of type 2 diabetes patients 23 , 58 . The measured serum amylin levels were high in individuals with chronic kidney disease 59 . This is ascribed to augmented insulin resistance in patients with end‐stage renal failure on dialysis treatment, which is associated with hypersecretion of amylin concurrently with reduced renal excretion of amylin. However, the increase in serum amylin was not directly associated with hypoinsulin secretion or an increase in insulin resistance, whereas amyloid deposition in the islets was pronounced in patients on hemodialysis. Although increased levels of circulating amylin are therefore unlikely to be a direct pathogenetic factor in the development of type 2 diabetes, increased amylin can contribute to the formation of amyloid in the islets.
AGING AND AMYLOID DEPOSITION
The prevalence of diabetes in the elderly is currently on the rise. Type 2 diabetes in the elderly can be divided into two types 60 . In one group, type 2 diabetes manifests in middle age and continues to old age, and in the other group, there is no metabolic disorder in middle age and diabetes first develops in old age. It is known that diabetes in the elderly has a different pathological condition from diabetes that develops in middle age 59 . For example, older‐onset type 2 diabetes is characterized by low involvement of genetic factors and high susceptibility to hypoglycemic attacks. In contrast, aging is a factor that enhances amyloid formation in the body 61 . For example, the accumulation of amyloid fibrils, such as amyloid‐β and amylin, plays key roles in the pathogenesis of fatal age‐related diseases, such as Alzheimer’s and Parkinson’s diseases 62 . The prevalence of amyloid deposition in the islets is increased with aging in Japanese individuals without diabetes 63 . A total of 6% of individuals without diabetes aged >80 years showed amyloid deposition in the islets 64 . Therefore, the exploration of the involvement of aging in the process of fibril accumulation in islets is extremely important in view of the increased life expectancy in our societies. It is also important to define the pathological and clinical characteristics of amyloid deposition in the elderly, which might help to explore the pathogenesis of islet amyloid deposition.
CHARACTERISTICS OF ISLET PATHOLOGY OF ELDERLY DIABETES PATIENTS
We evaluated the pathological changes of the islets in the autopsy pancreas samples of elderly type 2 diabetes patients 21 . The average age of the group was 88.0 ± 0.6 years, and the age at type 2 diabetes onset was 80.8 ± 1.4 years. BMI was comparable between individuals with and without diabetes. Pancreatic weight was reduced by half in the senile‐onset type 2 diabetes group compared with the elderly group without diabetes. Islet amyloid deposits were significantly increased in middle‐aged type 2 diabetes patients compared with age‐matched individuals without type 2 diabetes. Amyloid deposition was significantly increased in the senile individuals with diabetes group compared with the senile individuals without diabetes group (Figure 5a). A comparison between the middle‐aged diabetes patients group and the senile diabetes patients group also showed that the senile diabetes group exhibited marked amyloid deposits. Both β‐cell and α‐cell masses were also significantly reduced in senile diabetes patients compared with middle‐aged diabetes patients. Interestingly, in senile diabetes patients, pancreatic intraepithelial neoplasia (PanIN) was frequently found in the pancreatic duct (Figure 5b‐d). Acinar cells were atrophic and fibrotic around the duct complicated with PanIN (Figure 5c,d). PanIN is thought to result in outflow disorder of pancreatic juice, leading to localized pancreatitis, exocrine atrophy, fibrosis and weight loss of the pancreas. From the aforementioned results, it was considered that senile‐onset diabetes mellitus shows a unique pancreatic pathology along with amyloid deposition of the islets. The present results show that the pathogenesis of older‐onset type 2 diabetes shares some features with pancreatic diabetes. Ueberberg et al. reported that the prevalence of islets containing amyloid deposits was significantly higher in both diabetes due to exocrine pancreatic disorders compared with healthy individuals 30 . This suggests that PanIN‐induced pancreatic atrophy might accelerate amyloid deposits in older‐onset type 2 diabetes.
Figure 5.
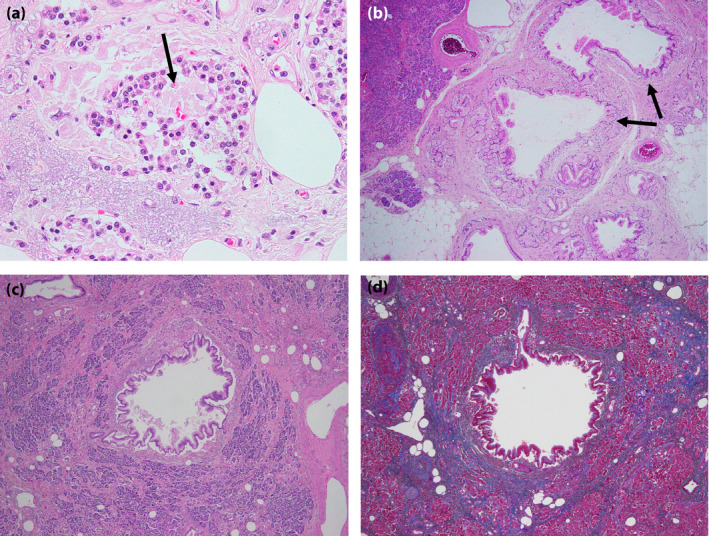
Morphological findings of the pancreas in older‐onset diabetes. (a) Marked amyloid deposits are observed in the islet (arrow). (b) In the same patient as (a), a few examples of intraepithelial neoplasia were observed (arrows). (c) In another patients, stromal fibrosis and atrophy of acinal cells surrounding an intraepithelial neoplasia were observed. (d) Stromal fibrosis in the exocrine pancreas was confirmed by Azan staining (blue).
In the present review, the diversity of islet pathological changes in human type 2 diabetes, especially in cases of amyloid deposits, and the similarity to subclassification of type 2 diabetes described by Ahlqvist et al. were shown (Table 1). The loss of β‐cell mass is a hallmark of the pathological change in islet endocrine cells in type 2 diabetes. However, a detailed pathological examination of the clinical course and cells other than β‐cells might clarify further pathophysiology and subclassification of type 2 diabetes. Recently, comprehensive molecular pathological analysis using paraffin blocks has become widespread in the field of cancer. We hope that the clinical and genetic classification of type 2 diabetes based on pathological exploration with such a new analysis method will be further advanced, and that the understanding of these pathological conditions and application of tailor‐made treatments will be established.
DISCLOSURE
The authors declare no conflict of interest.
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of Hirosaki University School of Medicine, date of approval: 20 October 2017, approval number #2017–121.
Informed consent: N/A.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
This study was supported in part by the Japanese Ministry of Education, Culture, Science and Sports, Grant‐in‐Aid for Scientific Research to HM (#18K08462). Technical assistance from Ms Saori Ogasawara, Misato Sakamoto, and Hiroko Mori of the Department of Pathology and Molecular Medicine of Hirosaki University Graduate School of Medicine was highly appreciated.
J Diabetes Investig 2022; 13: 6–13
REFERENCES
- 1. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population‐based studies with 4.4 million participants. Lancet 2016; 387: 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Fronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care 1992; 15: 318–368. [DOI] [PubMed] [Google Scholar]
- 3. Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835. [DOI] [PubMed] [Google Scholar]
- 4. Møller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014; 37: 796–804. [DOI] [PubMed] [Google Scholar]
- 5. Kou K, Saisho Y, Satoh S, et al. Change in β‐cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab 2013; 98: 3724–3730. [DOI] [PubMed] [Google Scholar]
- 6. Mizukami H, Takahashi K, Inaba W, et al. Age‐associated changes of islet endocrine cells and the effects of body mass index in Japanese. J Diabetes Investig 2014; 5: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yagihashi S, Inaba W, Mizukami H. Dynamic pathology of islet endocrine cells in type 2 diabetes: β‐Cell growth, death, regeneration and their clinical implications. J Diabetes Investig 2016; 7: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saisho Y, Butler AE, Manesso E, et al. β‐cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 2013; 36: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia 1978; 15: 417–421. [DOI] [PubMed] [Google Scholar]
- 10. Kamata K, Mizukami H, Inaba W, et al. Islet amyloid with macrophage migration correlates with augmented β‐cell deficits in type 2 diabetic patients. Amyloid 2014; 21: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult‐onset diabetes and their association with outcomes: a data‐driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018; 6: 361–369. [DOI] [PubMed] [Google Scholar]
- 12. Zaharia OP, Strassburger K, Strom A, et al. Risk of diabetes‐associated diseases in subgroups of patients with recent‐onset diabetes: a 5‐year follow‐up study. Lancet Diabetes Endocrinol 2019; 7: 684–694. [DOI] [PubMed] [Google Scholar]
- 13. Müller WA, Faloona GR, Aguilar‐Parada E, et al. Abnormal alpha‐cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 1970; 283: 109–115. [DOI] [PubMed] [Google Scholar]
- 14. Pfeifer MA, Halter JB, Porte D Jr. Insulin secretion in diabetes mellitus. Am J Med 1981; 70: 579–588. [DOI] [PubMed] [Google Scholar]
- 15. Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia 1983; 24: 366–371. [DOI] [PubMed] [Google Scholar]
- 16. Sakuraba H, Mizukami H, Yagihashi N, et al. Reduced beta‐cell mass and expression of oxidative stress‐related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002; 45: 85–96. [DOI] [PubMed] [Google Scholar]
- 17. Butler AE, Janson J, Soeller WC, et al. Increased beta‐cell apoptosis prevents adaptive increase in beta‐cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 2003; 52: 2304–2314. [DOI] [PubMed] [Google Scholar]
- 18. Zhao H‐L, Lai FM, Tong PC, et al. Prevalence and clinicopathological characteristics of islet amyloid in Chinese patients with type 2 diabetes. Diabetes 2003; 52: 2759–2766. [DOI] [PubMed] [Google Scholar]
- 19. Yoon KH, Ko SH, Cho JH, et al. Selective beta‐cell loss and alpha‐cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003; 88: 2300–2308. [DOI] [PubMed] [Google Scholar]
- 20. Mizukami H, Takahashi K, Inaba W, et al. Involvement of oxidative stress‐induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of β‐cell mass in Japanese type 2 diabetic patients. Diabetes Care 2014; 37: 1966–1974. [DOI] [PubMed] [Google Scholar]
- 21. Xin AN, Mizukami H, Inaba W, et al. Pancreas atrophy and islet amyloid deposition in patients with elderly‐onset type 2 diabetes. J Clin Endocrinol Metab 2017; 102: 3162–3171. [DOI] [PubMed] [Google Scholar]
- 22. Amo‐Shiinoki K, Tanabe K, Hoshii Y, et al. Islet cell dedifferentiation is a pathologic mechanism of long‐standing progression of type 2 diabetes. JCI Insight 2021; 6: e143791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi K, Mizukami H, Osonoi S, et al. Islet microangiopathy and augmented β‐cell loss in Japanese non‐obese type 2 diabetes patients who died of acute myocardial infarction. J Diabetes Investig. 2021. doi: 10.1111/jdi.13601. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rorsman P, Berggren P‐O, Bokvist K, et al. Glucose‐inhibition of glucagon secretion involves activation of GABAA‐receptor chloride channels. Nature 1989; 341: 23323–23326. [DOI] [PubMed] [Google Scholar]
- 25. Ishihara H, Maechler P, Gjinovci A, et al. Islet beta‐cell secretion determines glucagon release from neighbouring alpha‐cells. Nat Cell Biol 2003; 5: 330–335. [DOI] [PubMed] [Google Scholar]
- 26. Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 2009; 9: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez‐Diaz R, Dando R, Jacques‐Silva MC, et al. Alpha cells secrete acetylcholine as a non‐neuronal paracrine signal priming beta cell function in humans. Nat Med 2011; 17: 888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cinti F, Bouchi R, Kim‐Muller JY, et al. Evidence of β‐cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 2016; 101: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jurgens CA, Toukatly MN, Fligner CL, et al. β‐cell loss and β‐cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol 2011; 178: 2632–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ueberberg S, Nauck MA, Uhl W, et al. Islet amyloid in patients with diabetes due to exocrine pancreatic disorders, type 2 diabetes, and nondiabetic patients. J Clin Endocrinol Metab 2020; 105: 2595–2605. [DOI] [PubMed] [Google Scholar]
- 31. Opie EL. The relation oe diabetes mellitus to lesions of the pancreas. hyaline degeneration of the islands oe langerhans. J Exp Med 1901; 5: 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen YC, Taylor AJ, Verchere CB. Islet prohormone processing in health and disease. Diabetes Obes Metab 2018; 20: 64–76. [DOI] [PubMed] [Google Scholar]
- 33. Ogawa A, Harris V, McCorkle SK, et al. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J Clin Investig 1990; 85: 973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qi D, Cai K, Wang O, et al. Fatty acids induce amylin expression and secretion by pancreatic beta‐cells. Am J Physiol Endocrinol Metab 2010; 298: E99–E107. [DOI] [PubMed] [Google Scholar]
- 35. Sanke T, Bell GI, Sample C, et al. An islet amyloid peptide is derived from an 89‐amino acid precursor by proteolytic processing. J Biol Chem 1988; 263: 17243–17246. [PubMed] [Google Scholar]
- 36. Kahn SE, D'Alessio DA, Schwartz MW, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta‐cells. Diabetes 1990; 39: 634–638. [DOI] [PubMed] [Google Scholar]
- 37. Park K, Verchere CB. Identification of a heparin binding domain in the N‐terminal cleavage site of pro‐islet amyloid polypeptide. Implications for islet amyloid formation. J Biol Chem 2001; 276: 16611–16616. [DOI] [PubMed] [Google Scholar]
- 38. de Koning EJP, Bodkin NL, Hansen BC, et al. Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in beta‐cell population. Diabetologia 1993; 36: 378–384. [DOI] [PubMed] [Google Scholar]
- 39. Soeller WC, Janson J, Hart SE, et al. Islet amyloid‐associated diabetes in obese avy/a mice expressing human islet amyloid polypeptide. Diabetes 1998; 47: 743–750. [DOI] [PubMed] [Google Scholar]
- 40. Kautzky‐Willer A, Thomaseth K, Pacini G, et al. Role of islet amyloid polypeptide secretion in insulin‐resistant humans. Diabetologia 1994; 37: 188–194. [DOI] [PubMed] [Google Scholar]
- 41. Kailasam MT, Parmer RJ, Tyrell EA, et al. Circulating amylin in human essential hypertension: heritability and early increase in individuals at genetic risk. J Hypertens 2000; 18: 1611–1620. [DOI] [PubMed] [Google Scholar]
- 42. Despa S, Margulies KB, Chen LE, et al. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats. Circ Res 2012; 110: 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu M, Verma N, Peng X, et al. Hyperamylinemia Increases IL‐1β Synthesis in the Heart via Peroxidative Sarcolemmal Injury. Diabetes 2016; 65: 2772–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu M, Li N, Qu C, et al. Amylin deposition activates HIF1α and 6‐phosphofructo‐2‐kinase/fructose‐2, 6‐biphosphatase 3 (PFKFB3) signaling in failing hearts of non‐human primates. Commun Biol 2021; 4: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verma N, Liu M, Ly H, et al. Diabetic microcirculatory disturbances and pathologic erythropoiesis are provoked by deposition of amyloid‐forming amylin in red blood cells and capillaries. Kidney Int 2020; 97: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Novials A, Rodriguez‐Mañas L, Chico A, et al. Amylin and hypertension: association of an amylin ‐G132A gene mutation and hypertension in humans and amylin‐induced endothelium dysfunction in rats. J Clin Endocrinol Metab 2007; 92: 1446–1450. [DOI] [PubMed] [Google Scholar]
- 47. Gæde P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. [DOI] [PubMed] [Google Scholar]
- 48. Tesfaye S, Chaturvedi N, Eaton SEM, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
- 49. Feldman EL, Nave K‐A, Jensen TS, et al. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017; 93: 1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mizukami H, Osonoi S. Pathogenesis and molecular treatment strategies of diabetic neuropathy collateral glucose‐utilizing pathways in diabetic polyneuropathy. Int J Mol Sci 2020; 22: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brissova M, Shostak A, Fligner CL, et al. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J Histochem Cytochem 2015; 63: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Held F, Morris A, Pirici D, et al. Vascular basement membrane alterations and β‐amyloid accumulations in an animal model of cerebral small vessel disease. Clin Sci 2017; 131: 1001–1013. [DOI] [PubMed] [Google Scholar]
- 53. Dybala MP, Kuznetsov A, Motobu M, et al. Integrated pancreatic blood flow: bidirectional microcirculation between endocrine and exocrine pancreas. Diabetes 2020; 69: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dai C, Brissova M, Reinert RB, et al. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes 2013; 62: 4144–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Almaça J, Weitz J, Rodriguez‐Diaz R, et al. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 2018; 27: 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giroix M‐H, Irminger J‐C, Lacraz G, et al. Hypercholesterolaemia, signs of islet microangiopathy and altered angiogenesis precede onset of type 2 diabetes in the Goto‐Kakizaki (GK) rat. Diabetologia 2011; 54: 2451–2462. [DOI] [PubMed] [Google Scholar]
- 57. Leckström A, Björklund K, Permert J, et al. Renal elimination of islet amyloid polypeptide. Biochem Biophys Res Commun 1997; 239: 265–268. [DOI] [PubMed] [Google Scholar]
- 58. de Koning EJP, Fleming KA, Gray DWR, et al. High prevalence of pancreatic islet amyloid in patients with end‐stage renal failure on dialysis treatment. J Pathol 1995; 175: 253–258. [DOI] [PubMed] [Google Scholar]
- 59. Ludvik B, Clodi M, Kautzky‐Willer A, et al. Increased levels of circulating islet amyloid polypeptide in patients with chronic renal failure have no effect on insulin secretion. J Clin Investig 1994; 94: 2045–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001; 414: 782–787. [DOI] [PubMed] [Google Scholar]
- 61. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med 2003; 349: 583–596. [DOI] [PubMed] [Google Scholar]
- 62. Tasaki M, Lavatelli F, Obici L, et al. Age‐related amyloidosis outside the brain: a state‐of‐the‐art review. Ageing Res Rev 2021; 70: 101388. [DOI] [PubMed] [Google Scholar]
- 63. Su YU, Misumi Y, Ueda M, et al. The occurrence of islet amyloid polypeptide amyloidosis in Japanese subjects. Pancreas 2012; 41: 971–973. [DOI] [PubMed] [Google Scholar]
- 64. Clark A, Saad MF, Nezzer T, et al. Islet amyloid polypeptide in diabetic and non‐diabetic Pima Indians. Diabetologia 1990; 33: 285–289. [DOI] [PubMed] [Google Scholar]


