Abstract
Aims/Introduction
To investigate the association between cardiovascular autonomic neuropathy (CAN) assessed by the coefficient of variation of the R‐R interval and the reduction in the estimated glomerular filtration rate (eGFR) in patients with type 2 diabetes.
Materials and methods
This retrospective observational cohort study enrolled type 2 diabetes patients who had their coefficient of variation of the R‐R interval measured on an electrocardiogram from January 2005 to December 2018. CAN was defined using the reference coefficient of variation of the R‐R interval value based on age and sex. The primary outcome was set as a 40% eGFR decline from baseline. Regression analyses using the Cox proportional hazards model were carried out to evaluate the association.
Results
Of the 831 patients, 118 (14.2%) were diagnosed with CAN. In the analysis of the primary outcome, the median follow‐up period was 5.3 years, and 25 (21.2%) patients with CAN and 78 (10.9%) patients without CAN developed a 40% eGFR decline. In the univariate regression analysis, CAN was significantly associated with a 40% eGFR decline (hazard ratio 2.42, 95% confidence interval 1.54–3.80). In the multivariate analysis, CAN remained almost significant after adjusting for the prognostic risk factors for CAN and the decline in the renal function, and an interaction with proteinuria was found. In analyses for the interaction effect between CAN and proteinuria, the presence of CAN synergistically increased the risk of an eGFR decline in patients with macroproteinuria.
Conclusions
CAN strongly increased the risk of a 40% eGFR decline from baseline, especially in type 2 diabetes patients with macroproteinuria.
Keywords: Coefficient of variation of the R‐R interval, Cardiovascular autonomic neuropathy, Decline in the renal function
This study investigated the association between cardiovascular autonomic neuropathy assessed by the coefficient of variation of the R‐R interval and the reduction in the estimated glomerular filtration rate in patients with type 2 diabetes. Cardiovascular autonomic neuropathy strongly increased the risk of a 40% estimated glomerular filtration rate decline from baseline, especially in patients with macroproteinuria.

INTRODUCTION
Diabetes is a major cause of end‐stage renal disease 1 . The natural history of renal dysfunction in diabetes patients has been classified into several stages: hyperfiltration, microproteinuria (microalbuminuria), macroproteinuria, reduction in the estimated glomerular filtration rate (eGFR) and, finally, end‐stage renal disease 2 . However, recent studies have shown that some diabetes patients develop eGFR loss at the normoproteinuria and microproteinuria stages 3 , 4 . In addition, an impaired renal function is associated with cardiovascular disease morbidity and mortality 5 , 6 . Therefore, identifying patients at high risk for a reduction in their eGFR is clinically important to improve their prognosis. At present, several risk factors associated with a decline in the renal function have been proposed, and proteinuria is generally known to be a strong risk factor for a reduction in the eGFR in patients with diabetes 7 .
Cardiovascular autonomic neuropathy (CAN) is a common and potentially underdiagnosed complication in patients with diabetes. Its prevalence reportedly ranges from 37% to 73% among type 2 diabetes patients 8 , 9 . The clinical signs of CAN are related to systemic circulation disorders, such as asymptomatic abnormality of heart rate, resting tachycardia (90–130 b.p.m.) and orthostatic hypotension. Furthermore, CAN was also reported to be associated with cardiovascular disease morbidity and mortality 10 , 11 . Hence, the development of CAN is therefore suggested to be related to other diabetic vascular complications.
Cardiac autonomic nerve activity strongly depends on an individual’s age and sex 12 , 13 , so the evaluation of the autonomic nerve activity needs to take into account these details. The cardiac autonomic nerve function can be assessed based on the heart rate variability, such as the results of the cardiovascular autonomic reflex test, a time domain analysis, and a power spectrum analysis 14 , 15 . The coefficient of the variation of the R‐R interval (CVR‐R) is one of the most convenient, non‐invasive tests of heart rate variability, and is widely used to assess cardiac autonomic nerve function in patients with diabetes. Several studies have shown the association between CAN and a decline in renal function 16 , 17 . However, most previous studies have used methods other than CVR‐R to diagnose CAN, and the staging of renal function was limited.
Given the aforementioned, the present study investigated the association between CAN assessed by CVR‐R and eGFR decline, as well as the interaction between CAN and the stages of renal function in patients with type 2 diabetes.
MATERIALS AND METHODS
Study design and participants
The present study was a retrospective single‐center observational cohort study in type 2 diabetes outpatients in the Institute for Medical Science, Asahi Life Foundation, Tokyo, Japan. This study was approved by the Committee of Ethics at this institution (approval number: 08702‐1).
A total of 3,400 diabetes outpatients who received treatments from January 2005 to December 2015 were enrolled in the present study. The inclusion criteria were as follows: (i) type 2 diabetes; (ii) CVR‐R measured from January 2005 to December 2018; (iii) serum creatinine level measured within −84 to 28 days of the CVR‐R measurement; (iv) serum creatinine level measured more than twice during follow up; and (v) follow‐up period >28 days. The exclusion criteria were as follows: (i) missing data; (ii) uncalculated CVR‐R because of arrythmia during measurement; (iii) a diagnosis of autonomic nervous system diseases other than diabetic neuropathy; (iv) a diagnosis of kidney disease (immunoglobulin A nephropathy, chronic glomerulonephritis, renal tuberculosis, renal infarction and antineutrophil cytoplasmic antibodies‐associated glomerulonephritis); and (v) outcome occurring within 28 days.
Clinical and laboratory data
The following data were collected at the first visit 18 : sex, age (years), diabetes duration (years), body mass index (BMI; kg/m2), glycated hemoglobin (HbA1c; %), standard deviation (SD) of the R‐R interval (ms), mean of the R‐R interval (ms), CVR‐R (%), heart rate (b.p.m.), serum creatinine (mg/dL), eGFR (mL/min/1.73 m2), urine albumin‐to‐creatinine ratio (ACR; mg/gCr), dipstick urine test, systolic blood pressure (SBP; mmHg), diastolic blood pressure (mmHg), total cholesterol (mg/dL), triglyceride (mg/dL), low‐density lipoprotein cholesterol (mg/dL), high‐density lipoprotein cholesterol (mg/dL), uric acid (UA; mg/dL), prevalence of neuropathic symptoms (numbness or neuralgia), prevalence of decreased distal sensation (vibration perception threshold by 128‐Hz tuning fork ≤10 s), prevalence of decreased or absent Achilles tendon reflex, prevalence of diabetic peripheral sensory neuropathy (DPSN), smoking status, prevalence of hypertension, prevalence of dyslipidemia, use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) and use of statins.
The BMI was calculated by dividing the weight (kg) by the square of height (m). The SD of the R‐R interval, mean of the R‐R interval and heart rate were derived from 100 continuous resting heart rates recorded by an oscillometry‐based device (BP‐203RPE III; Omron Colin, Co., Ltd., Tokyo, Japan). During the measurement, patients were required to rest for 10–15 min in the supine position. The CVR‐R was calculated using the following equation: CVR‐R = SD of the R‐R interval / mean of the R‐R interval × 100. The eGFR was calculated using the following equation advocated by the Japanese Society of Nephrology: eGFR = 194 × serum creatinine−1.094 × age−0.287 (female ×0.739) 19 . Proteinuria was assessed using the dipstick urine test and/or ACR as albuminuria, and values were divided into three groups: (i) normoproteinuria for a dipstick urine test (−) and ACR <30 mg/gCr; (ii) microproteinuria for a dipstick urine test (±) or ACR 30 to <300 mg/gCr; and (iii) macroproteinuria for a dipstick urine test ≥(1+) or ACR ≥300 mg/gCr 20 . DPSN was defined as two or more of the following symptoms or signs: neuropathic symptoms (numbness or neuralgia), decreased distal sensation (vibration perception threshold by 128‐Hz tuning fork ≤10 s) or decreased or absent Achilles tendon reflex 21 . Patients with neuropathies other than diabetic neuropathy were excluded from the DPSN evaluation. Hypertension was defined as an SBP ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or the use of antihypertensive medications. Dyslipidemia was defined as triglyceride ≥150 mg/dL and/or low‐density lipoprotein cholesterol ≥140 mg/dL and/or high‐density lipoprotein cholesterol <40 mg/dL and/or the use of antihyperlipidemic medications. All eGFR data were followed during the visit.
CAN assessment
CAN was defined using the reference values of CVR‐R based on age and sex advocated by Agelink et al. 22 The reference values of CVR‐R were as follows: men: 3.05 (aged 20–24 years), 2.80 (aged 25–29 years), 2.58 (aged 30–34 years), 2.37 (aged 35–39 years), 2.18 (aged 40–44 years), 2.00 (aged 45–49 years), 1.88 (aged 50–54 years), 1.69 (aged 55–59 years), 1.55 (aged 60–64 years) and 1.43 (aged >65 years); women: 2.57 (aged 20–24 years), 2.38 (aged 25–29 years), 2.20 (aged 30–34 years), 2.04 (aged 35–39 years), 1.88 (aged 40–44 years), 1.74 (aged 45–49 years), 1.61 (aged 50–54 years), 1.49 (aged 55–59 years), 1.38 (aged 60–64 years) and 1.28 (aged >65 years). If the aforementioned CAN criteria were met, the patients were referred to as CAN (+); otherwise, they were referred to as CAN (−).
Outcomes
The primary outcome was defined as the first 40% eGFR decline from baseline. Recently, a 40% eGFR decline was used as the outcome to the assess renal function in an observational study 23 . In addition, two secondary outcomes: a 30% eGFR decline from baseline and obtaining an eGFR <60 mL/min/1.73 m2, were identified based on sensitivity analyses to confirm the robustness of the results for the primary outcome.
Statistical analysis
Regarding the baseline values, continuous variables showing a normal or highly skewed distribution are represented as the mean ± SD or median with interquartile range (IQR), respectively. Categorical variables are represented as the number with the percentage (%). To test the baseline difference between patients with CAN (−) and CAN (+), unpaired Student’s t‐tests was carried out for continuous variables. For highly skewed continuous variables, Wilcoxon’s rank sum test was carried out. For categorical variables, χ2‐tests or Fisher’s exact test were carried out. In the subgroup analysis, Dunnett’s test and Steel’s test were carried out to evaluate the baseline differences between the reference group of CAN (−) normoproteinuria and other multiple groups. We used the Kaplan–Meier method to assess the effect of CAN on the reduction in the eGFR, and carried out a log‐rank test to compare the survival rate among groups.
Furthermore, univariate and multivariate regression analyses using the Cox proportional hazards model were carried out as previously described 24 to assess whether or not CAN was associated with a decline in the renal function. In the univariate analysis, we evaluated the association between the eGFR decline and the following factors: CAN, DPSN, proteinuria, diabetes duration, BMI, HbA1c, SBP, triglyceride, UA, smoking status and use of ACEIs/ARBs. In the multivariate analysis, model 1 was used to evaluate the confounding effect between CAN and DPSN. We then evaluated whether or not CAN was independently associated with the eGFR decline in model 2 after adjusting for the following prognostic risk factors for CAN 25 , 26 and the decline in the renal function 24 , 27 , 28 , 29 : proteinuria, diabetes duration, BMI, HbA1c, SBP, triglyceride, UA, smoking status and use of ACEIs/ARBs.
In addition, we also carried out univariate and multivariate regression analyses using the Cox proportional hazards model to investigate the interaction effect between CAN and proteinuria. These results were represented as the hazard ratio (HR) with 95% confidence intervals (CIs).
A P‐value <0.05 was considered statistically significant. Analyses were carried out using the JMP® Pro 14 software program (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics
Among 3,400 patients in our database, 859 were eligible according to the inclusion criteria. Among those patients, 28 patients were excluded, and 831 patients were ultimately included in the analysis for the primary outcome (Figure 1). For the secondary analyses, 828 patients with a 30% eGFR decline from baseline and 722 with an eGFR <60 mL/min/1.73 m2 were included.
Figure 1.
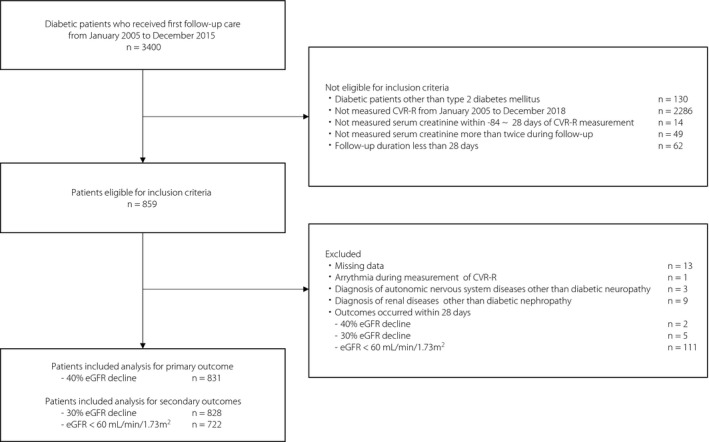
Inclusion and exclusion criteria in this study. In the analysis for the primary outcome (40% estimated glomerular filtration rate [eGFR] decline from baseline), 831 patients were included. In the analysis for the secondary outcomes (30% eGFR decline from baseline and eGFR <60 mL/min/1.73 m2), 828 and 722 patients were included, respectively. CVR‐R: coefficient of variation of the R‐R interval.
Table 1 shows the clinical and laboratory characteristics of 831 patients (608 men: 73.2%, 223 women: 26.8%). The mean age was 55.9 ± 11.6 years. The median diabetes duration was 3 years (IQR 0–9 years). The mean BMI, HbA1c and eGFR were 25.8 ± 4.9 kg/m2, 8.6 ± 2.0% and 80.3 ± 20.9 mL/min/1.73 m2, respectively. Of the 831 patients, 713 (85.8%) and 118 (14.2%) were diagnosed as CAN (−) and CAN (+). Compared with CAN (−) patients, CAN (+) patients had a higher proportion of women (P = 0.01), longer diabetes duration (P = 0.02), higher serum creatinine level (P = 0.046), higher prevalence of proteinuria (P = 0.0007), higher SBP (P = 0.0003), higher diastolic blood pressure (P = 0.03), higher triglyceride (P = 0.049), higher UA (P = 0.02), higher prevalence of decreased or absent Achilles tendon reflex (P = 0.0002), higher prevalence of smoking status (P = 0.02), higher prevalence of hypertension (P = 0.003) and higher prevalence of dyslipidemia (P = 0.048).
Table 1.
Baseline characteristics of 831 patients with type 2 diabetes
| Total | CAN (−) | CAN (+) | P‐value | |
|---|---|---|---|---|
| n = 831 | n = 713 | n = 118 | ||
| Women, n (%) | 223 (26.8) | 202 (28.3) | 21 (17.8) | 0.01 |
| Age (years) | 55.9 ± 11.6 | 55.9 ± 11.6 | 56.4 ± 11.4 | 0.65 |
| Diabetes duration (years) | 3 (0, 9) | 3 (0, 9) | 4 (0, 11) | 0.02 |
| Body mass index (kg/m2) | 25.8 ± 4.9 | 25.7 ± 4.9 | 26.0 ± 4.6 | 0.64 |
| HbA1c (%) | 8.6 ± 2.0 | 8.6 ± 2.0 | 8.6 ± 1.8 | 0.93 |
| Unknown, n (%) | 4 (0.5) | 3 (0.4) | 1 (0.8) | |
| Standard deviation of R‐R interval (ms) | 26.4 ± 13.5 | 29.0 ± 12.8 | 10.8 ± 3.7 | <0.0001 |
| Mean of R‐R interval (ms) | 880.2 ± 135.9 | 889.3 ± 132.6 | 825.3 ± 143.0 | <0.0001 |
| CVR‐R (%) | 3.0 ± 1.4 | 3.2 ± 1.3 | 1.3 ± 0.39 | <0.0001 |
| Heart rate (b.p.m.) | 61.4 ± 12.8 | 61.1 ± 12.0 | 63.4 ± 16.8 | 0.07 |
| Serum creatinine (mg/dL) | 0.80 ± 0.43 | 0.79 ± 0.42 | 0.88 ± 0.46 | 0.046 |
| eGFR (mL/min/1.73 m2) | 80.3 ± 20.9 | 80.8 ± 20.4 | 77.4 ± 24.0 | 0.11 |
| Urine albumin‐to‐creatinine‐ratio (mg/gCr) | 9.8 (5.4–24.8) | 9.7 (5.1–23.6) | 12.4 (5.8–42.7) | 0.052 |
| Unknown, n (%) | 167 (20.1) | 132 (18.5) | 35 (29.7) | |
| Dipstick urine test | ||||
| (−)/(±)/(1+, 2+, 3+), n | 706/32/92 | 620/26/67 | 86/6/25 | 0.0007 |
| (−)/(±)/(1+, 2+, 3+), % | 85.1/3.9/11.0 | 87.0/3.6/9.4 | 73.5/5.1/21.4 | |
| Unknown, n (%) | 1 (0.1) | – | 1 (0.8) | |
| Systolic blood pressure (mmHg) | 130.9 ± 17.6 | 130.0 ± 17.0 | 136.3 ± 20.1 | 0.0003 |
| Unknown, n (%) | 11 (1.3) | 9 (1.3) | 2 (1.7) | |
| Diastolic blood pressure (mmHg) | 77.5 ± 11.4 | 77.2 ± 11.2 | 79.7 ± 12.8 | 0.03 |
| Unknown, n (%) | 11 (1.3) | 9 (1.3) | 2 (1.7) | |
| Total cholesterol (mg/dL) | 196.8 ± 37.6 | 196.2 ± 36.7 | 200.5 ± 42.6 | 0.25 |
| Triglyceride (mg/dL) | 127 (89, 181) | 126 (88, 178) | 147 (95, 191) | 0.049 |
| Unknown, n (%) | 1 (0.1) | 1 (0.1) | – | |
| LDL cholesterol (mg/dL) | 114.9 ± 32.4 | 114.7 ± 32.2 | 116.0 ± 33.4 | 0.71 |
| Unknown, n (%) | 92 (11.1) | 82 (11.5) | 10 (8.5) | |
| HDL cholesterol (mg/dL) | 50.3 ± 14.1 | 50.5 ± 13.9 | 48.5 ± 14.8 | 0.14 |
| Unknown, n (%) | 2 (0.2) | 1 (0.1) | 1 (0.8) | |
| Uric acid (mg/dL) | 5.6 ± 1.4 | 5.6 ± 1.4 | 5.9 ± 1.5 | 0.02 |
| Unknown, n (%) | 2 (0.2) | 2 (0.3) | – | |
| Neuropathic symptoms, n (%) | 134 (23.1) | 117 (23.3) | 17 (21.8) | 0.77 |
| Unknown, n (%) | 240 (29.2) | 200 (28.4) | 40 (33.9) | |
| Decreased distal sensation, n (%) | 248 (40.4) | 211 (39.7) | 37 (45.1) | 0.35 |
| Unknown, n (%) | 207 (25.2) | 171 (24.3) | 36 (30.5) | |
| Decreased or absent ATR, n (%) | 309 (48.4) | 253 (45.5) | 56 (67.5) | 0.0002 |
| Unknown, n (%) | 182 (22.2) | 147 (20.9) | 35 (29.7) | |
| DPSN, n (%) | 158 (32.2) | 134 (31.2) | 24 (39.3) | 0.21 |
| Unknown, n (%) | 331 (40.3) | 274 (39.0) | 57 (48.3) | |
| Smoking status, n (%) | 477 (57.4) | 398 (55.8) | 79 (66.9) | 0.02 |
| Hypertension, n (%) | 318 (38.3) | 258 (36.2) | 60 (50.8) | 0.003 |
| Dyslipidemia, n (%) | 488 (58.7) | 409 (57.4) | 79 (66.9) | 0.048 |
| Use rate of ACEIs/ARBs, n (%) | 109 (13.1) | 88 (12.3) | 21 (17.8) | 0.12 |
| Use rate of statin, n (%) | 56 (6.7) | 47 (6.6) | 9 (7.6) | 0.68 |
The values were represented as the mean ± standard deviation, median with interquartile range or number with percentage (%). To test the significance between cardiovascular autonomic neuropathy (CAN) (−) and CAN (+) patients, we used the unpaired Student’s t‐tests for continuous variables, Wilcoxon’s rank sum test for highly skewed continuous variables and the χ2‐test or Fisher's exact test for categorical variables. Statistical significance was defined as a P‐value <0.05.
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; ATR, Achilles tendon reflex; CVR‐R, coefficient of variation of the R‐R interval; DPSN, diabetic peripheral sensory neuropathy; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Kaplan–Meier analyses comparing CAN (−) and CAN (+) patients with a 40% eGFR decline
Figure 2a shows the results of a Kaplan–Meier analysis for a 40% eGFR decline in patients stratified by the presence of CAN. The median follow‐up period was 5.3 years (range 0–13 years). A total of 103 (12.4%) of the 831 patients showed a 40% eGFR decline during follow up. A total of 78 (10.9%) CAN (−) patients and 25 (21.2%) CAN (+) patients developed a 40% eGFR decline. CAN (+) patients were more likely to show a reduced eGFR than CAN (−) patients (log‐rank test, P < 0.0001).
Figure 2.
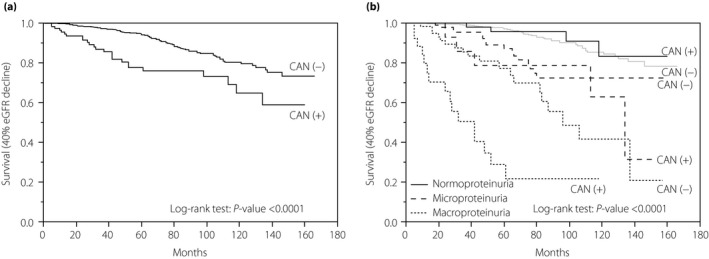
A Kaplan–Meier analysis for a 40% estimated glomerular filtration rate (eGFR) decline from baseline. (a) Kaplan–Meier curves showed the survival after a 40% eGFR decline among the 831 type 2 diabetes cardiovascular autonomic neuropathy (CAN) (−) and CAN (+) patients. The median follow‐up period was 5.3 years. Among the total 831 patients, 103 (12.4%) developed a 40% eGFR decline from baseline. Among CAN (−) and CAN (+) patients, 78 (10.9%) and 25 (21.2%) developed a 40% eGFR decline, respectively (log‐rank test: P < 0.0001). (b) Kaplan–Meier curves carried out the survival after a 40% eGFR decline in 830 type 2 diabetes patients stratified by CAN and proteinuria. Patients were divided into six groups: (i) CAN (−) normoproteinuria (n = 539); (ii) CAN (+) normoproteinuria (n = 73); (iii) CAN (−) microproteinuria (n = 106); (iv) CAN (+) microproteinuria (n = 17); (v) CAN (−) macroproteinuria (n = 68); and (vi) CAN (+) macroproteinuria (n = 27). Proteinuria was defined as follows: normoproteinuria: dipstick urine test (−) and urine albumin‐to‐creatinine ratio <30 mg/gCr; microproteinuria: dipstick urine test (±) or urine albumin‐to‐creatinine ratio 30 to <300 mg/gCr; macroproteinuria: dipstick urine test ≥(1+) or urine albumin‐to‐creatinine ratio ≥300 mg/gCr. Log‐rank test: P < 0.0001.
Kaplan–Meier analyses in patients stratified by CAN and proteinuria
Figure 2b shows the results of a Kaplan–Meier analysis for a 40% eGFR decline in patients divided into six groups: (i) CAN (−) normoproteinuria (n = 539); (ii) CAN (+) normoproteinuria (n = 73); (iii) CAN (−) microproteinuria (n = 106); (iv) CAN (+) microproteinuria (n = 17); (v) CAN (−) macroproteinuria (n = 68); and (vi) CAN (+) macroproteinuria (n = 27). A 40% eGFR decline from baseline developed in 43 (8.0%) CAN (−) and four (5.5%) CAN (+) patients with normoproteinuria, 16 (15.1%) CAN (−) and five (29.4%) CAN (+) patients with microproteinuria, and 19 (27.9%) CAN (−) and 16 (59.3%) CAN (+) patients with macroproteinuria (log‐rank test, P < 0.0001).
The results of a Kaplan–Meier analysis for sensitivity analyses (30% eGFR decline from baseline and eGFR <60 mL/min/1.73 m2) are shown in Figures S1 and S2. In the analysis for the 30% decline in eGFR from baseline, 208 (25.2%) of 827 patients developed the outcome. A 30% eGFR decline from baseline developed in 106 (19.7%) CAN (−) and 15 (20.5%) CAN (+) patients with normoproteinuria, 34 (32.1%) CAN (−) and eight (47.1%) CAN (+) patients with microproteinuria, and 30 (44.1%) CAN (−) and 15 (60.0%) CAN (+) patients with macroproteinuria (log‐rank test, P < 0.0001). In the analysis for an eGFR <60 mL/min/1.73 m2, 226 (31.3%) of 721 patients developed the outcome. An eGFR <60 mL/min/1.73 m2 developed in 150 (30.5%) CAN (−) and 17 (26.6%) CAN (+) patients with normoproteinuria, 31 (34.1%) CAN (−) and six (37.5%) CAN (+) patients with microproteinuria, and 13 (28.9%) CAN (−) and nine (64.3%) CAN (+) patients with macroproteinuria (log‐rank test, P < 0.0001).
Kaplan–Meier analyses in patients stratified by CAN and DPSN
In Figure S3, the result of Kaplan–Meier analysis for the 40% decline in eGFR from baseline in patients stratified by CAN and DPSN is shown. A total of 331 patients were unmeasured neuropathic findings for diagnosis of DPSN at baseline. The number of respective groups was as follows: unidentified (n = 331); CAN (−) DPSN (−) (n = 295); CAN (−) DPSN (+) (n = 134); CAN (+) DPSN (−) (n = 37); and CAN (+) DPSN (+) (n = 24). A 40% eGFR decline from baseline developed in 35 (10.6%) unidentified patients, 26 (8.8%) DPSN (−) patients and 27 (20.1%) DPSN (+) patients with CAN (−), and eight (21.6%) DPSN (−) patients and five (20.8%) DPSN (+) patients with CAN (+). The diagnosis of either CAN or DSPN worsened the renal prognosis (log‐rank test, P = 0.04).
Regression analyses using a Cox proportional hazards model
Table 2 shows the results of univariate and multivariate regression analyses using a Cox proportional hazards model. In the univariate analysis, a 40% eGFR decline from baseline was significantly associated with CAN (HR 2.42, 95% CI 1.54–3.80), DPSN (HR 1.67, 95% CI 1.03–2.72), microproteinuria (HR 2.76, 95% CI 1.65–4.62), macroproteinuria (HR 9.77, 95% CI 6.26–15.3), diabetes duration (HR 1.03, 95% CI 1.01–1.05), SBP (HR 1.03, 95% CI 1.02–1.04), triglyceride (HR 1.003, 95% CI 1.002–1.004) and UA (HR 1.30, 95% CI 1.15–1.47).
Table 2.
Results of univariate and multivariate regression analyses using the Cox proportional hazards model for a 40% estimated glomerular filtration rate decline
| Univariate | Multivariate (model 1) | Multivariate (model 2) | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| CAN | 2.42 (1.54–3.80) | 0.0001 | 1.91 (1.04–3.51) | 0.04 | 1.54 (0.94–2.51) | 0.09 |
| DPSN | 1.67 (1.03–2.72) | 0.04 | 1.66 (1.02–2.70) | 0.04 | ||
| Proteinuria | ||||||
| Normoproteinuria | 1.00 (Reference) | – | 1.00 (Reference) | – | ||
| Microproteinuria | 2.76 (1.65–4.62) | 0.0001 | 2.62 (1.54–4.48) | 0.0004 | ||
| Macroproteinuria | 9.77 (6.26–15.3) | <0.0001 | 6.82 (4.08–11.4) | <0.0001 | ||
| Diabetes duration (years) | 1.03 (1.01–1.05) | 0.04 | 1.02 (0.99–1.05) | 0.14 | ||
| Body mass index (kg/m2) | 1.02 (0.98–1.06) | 0.26 | 0.94 (0.90–0.99) | 0.02 | ||
| HbA1c (%) | 1.05 (0.96–1.14) | 0.24 | 1.04 (0.94–1.15) | 0.45 | ||
| Systolic blood pressure (mmHg) | 1.03 (1.02–1.04) | <0.0001 | 1.02 (1.01–1.03) | 0.02 | ||
| Triglyceride (mg/dL) | 1.003 (1.002–1.004) | <0.0001 | 1.002 (1.001–1.003) | <0.0001 | ||
| Uric acid (mg/dL) | 1.30 (1.15–1.47) | <0.0001 | 1.21 (1.04–1.40) | 0.01 | ||
| Smoking status | 0.99 (0.67–1.46) | 0.97 | 0.89 (0.58–1.36) | 0.58 | ||
| Use of ACEIs/ARBs | 1.44 (0.86–2.43) | 0.17 | 1.03 (0.58–1.82) | 0.92 | ||
The results of univariate and multivariate regression analyses using the Cox proportional hazards model were represented as hazard ratios with 95% confidence intervals.
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CAN, cardiovascular autonomic neuropathy; CI, confidence interval; DPSN, diabetic peripheral sensory neuropathy; HbA1c, glycated hemoglobin.
In the multivariate analyses, we assessed the confounding effect between CAN and DPSN in model 1 and whether or not CAN was independently associated with a 40% eGFR decline in model 2. In model 1, CAN was independently associated with a 40% eGFR decline, even if DPSN was incorporated into the model simultaneously (HR 1.91, 95% CI 1.04–3.51). In contrast, CAN was not independently associated with a 40% eGFR decline in model 2 (HR 1.54, 95% CI 0.94–2.51).
Evaluation of the interaction effect between CAN and proteinuria on a 40% eGFR decline
Table 3 shows the unadjusted and adjusted HRs for a 40% eGFR decline derived from the regression analysis using a Cox proportional hazards model to investigate the interaction effect between CAN and proteinuria. In the unadjusted analysis, an interaction effect for a 40% eGFR decline was found in patients with microproteinuria and macroproteinuria: CAN (−) microproteinuria (HR 2.51, 95% CI 1.41–4.45); CAN (+) microproteinuria (HR 3.73, 95% CI 1.48–9.43); CAN (−) macroproteinuria (HR 6.36, 95% CI 3.69–11.0); CAN (+) macroproteinuria (HR 26.4, 95% CI 14.5–47.9). In the adjusted analysis, after adjusting for the prognostic risk factors for CAN and/or a decline in the renal function (diabetes duration, BMI, HbA1c, SBP, triglyceride, UA, smoking status and use of ACEIs or ARBs), the interaction effect remained statistically significant in macroproteinuria: CAN (−) macroproteinuria (HR 5.24, 95% CI 2.93–9.37); CAN (+) macroproteinuria (HR 14.8, 95% CI 7.03–31.2). As a result, the presence of CAN likely multiplies the risk for a decline in the eGFR as the severity of proteinuria increases. In contrast, the risk of an eGFR decline was not significantly different between CAN (−) and CAN (+) in patients with normoproteinuria (HR 0.75, 95% CI 0.27–2.10).
Table 3.
Results of unadjusted and adjusted regression analyses using the Cox proportional hazards model to investigate the interaction effect between cardiovascular autonomic neuropathy and proteinuria
| Normoproteinuria | Microproteinuria | Macroproteinuria | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Unadjusted | ||||||
| CAN (−) | 1.00 (Reference) | – | 2.51 (1.41–4.45) | 0.002 | 6.36 (3.69–11.0) | <0.0001 |
| CAN (+) | 0.81 (0.29‐2.26) | 0.69 | 3.73 (1.48–9.43) | 0.005 | 26.4 (14.5–47.9) | <0.0001 |
| Adjusted | ||||||
| CAN (−) | 1.00 (Reference) | – | 2.53 (1.40–4.58) | 0.002 | 5.24 (2.93–9.37) | <0.0001 |
| CAN (+) | 0.75 (0.27–2.10) | 0.58 | 3.27 (1.27–8.45) | 0.01 | 14.8 (7.03–31.2) | <0.0001 |
The results were adjusted for the following factors: diabetes duration, body mass index, glycated hemoglobin, systolic blood pressure, triglyceride, uric acid, smoking status and use of angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers. Values were represented as hazard ratios with 95% confidence intervals (CI).
CAN, cardiovascular autonomic neuropathy.
DISCUSSION
In the present study, we showed the interaction effect between CAN and proteinuria on the eGFR decline in patients with type 2 diabetes. CAN synergistically influenced the reduction in the eGFR in patients with macroproteinuria. In contrast, the HR for a 40% eGFR decline was not significantly different between patients with and without CAN among those with normoproteinuria.
Previous studies have shown that CAN was associated with the development of chronic kidney disease (CKD) and a reduction in the eGFR among patients with type 2 diabetes 16 , 17 . However, these studies include patients with limited disease stages, and did not refer to the interaction effect between CAN and proteinuria on the decline in the renal function. Specifically, these previous studies defined the primary outcome as development of CKD (eGFR < 60 mL/min/1.73 m2 or albuminuria). In the present study, the primary outcome was defined as 40% eGFR decline to assess patients with the extensive ranges of renal function at baseline.
Furthermore, these previous studies defined CAN using the Ewing method as follows: excitation/inhibition ratio, 30:15 ratio and Valsalva ratio 15 , 16 , 17 . In Japan, the CVR‐R is routinely used to assess the cardiac autonomic nerve activity in patients with diabetes 30 , in addition to a 12‐lead electrocardiogram simultaneously obtained without physical or economic burden on the patients. CVR‐R is more convenient and easier to carry out for the evaluation of the cardiovascular autonomic activity than the Ewing method. However, definite diagnostic criteria of the CVR‐R have not yet been established. Thus, various diagnosis methods and criteria have been used in studies focused on CAN. In addition, the cardiovascular autonomic nerve function is known to differ by age and sex 12 , 13 , 31 . Therefore, we used reference values of CVR‐R based on age and sex 22 .
As with the Ewing method (excitation/inhibition ratio, 30:15 ratio and Valsalva ratio), the CVR‐R reflects the parasympathetic nerve activity 30 , 32 . Regarding autonomic nerve dysfunction, it is generally known that diabetic neuropathy initially affects the longer nerve fibers, such as the parasympathetic nerve 33 . The sympathetic nerve function relatively increases as the parasympathetic nerve function decreases 34 . Overactivity of sympathetic nerves results in changes in the renal hemodynamics, such as the absence of nocturnal blood pressure dipping 35 . The absence of nocturnal blood pressure dipping places strain on the kidney and causes a kidney injury‐induced decline in the renal function 36 . In the present study, although DSPN were not fully evaluated in all patients at baseline, CAN without DSPN evaluated at baseline, as well as with DSPN, showed deterioration of renal function. The early diagnosis and prevention of CAN might thus be associated with reducing the kidney burden in patients with diabetes.
In contrast, CKD itself might contribute to development of CAN. The patients with impaired renal function have lower autonomic nerve activity 37 . Furthermore, renal transplantation has recovered autonomic nerve function in patients with end‐stage renal disease 38 . For these reasons, autonomic dysfunction and deterioration of renal function could adversely affect each other and worsen renal function. Although several mechanisms are proposed, the exact mechanisms contributing to deterioration of autonomic nerve function are not clarified in patients with CKD 39 .
CAN is one of the most common diabetic complications in the early stage of diabetes. Nevertheless, ways to prevent and manage CAN are limited, typically involving blood glucose control, management of risk factors related to CAN progression and the use of aldose reductase inhibitors 40 , 41 , 42 . Previous studies have shown that intensive blood glucose control and the management of risk factors for CAN delayed the development and progression of the decline in the cardiac autonomic function 40 , 41 . However, the positive effects of aldose reductase inhibitors for autonomic neuropathy appear to be limited to patients with good glycemic control and no or mild microangiopathies 42 . Thus, there are few effective pharmacological interventions at the various stage of neuropathy available at present. Further basic and clinical studies are required to identify more effective pharmacological treatments. To prevent the development and progression of CAN, blood glucose control and the management of risk factors for CAN are important for patients with diabetes. In addition, physicians should routinely measure CVR‐R in daily clinical practice to detect patients with early‐stage neuropathy.
Several limitations associated with the present study warrant mention. First, the present study was a single‐center observational study, and the number of patients, particularly those with microproteinuria and macroproteinuria, was not large; this might have resulted in selection bias. In addition, selection bias might also exist because not all of the patients had their CVR‐R measured. However, why the CVR‐R was not measured was not clarified. Most measurements of CVR‐R do not seem to be arbitrary, because the measurements were routinely carried out at the first visit in addition to a 12‐lead electrocardiogram for patient with diabetes, if the electrocardiogram device also measured the CVR‐R. Second, the diagnostic criteria of CAN in the present study have previously been used based on data obtained from 309 healthy subjects 22 . To accurately detect the presence of cardiac autonomic dysfunction in patients with diabetes, large‐scale research targeted diabetes patients with a wide age range will be required. Finally, we defined proteinuria using the dipstick urine test and ACR. The dipstick urine test is a semiquantitative method of detecting proteinuria, whereas the ACR is a quantitative method of detecting albuminuria. The ACR was able to detect patients with microproteinuria who could not be detected by the dipstick urine test. However, due to health insurance issues in Japan, the ACR is generally not measured in clinical practice in patients with macroproteinuria and not frequently measured in patients with normoproteinuria.
Despite the aforementioned limitations, the present results shed light on the importance of diagnosing CAN to detect the high risk for eGFR decline of patients with type 2 diabetes. Future studies about CAN might clarify the way to prevent diabetic renal dysfunction.
The presence of CAN assessed by the CVR‐R and proteinuria synergistically increased the risk of eGFR decline in patients with type 2 diabetes.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Figure S1| Results of a Kaplan–Meier analysis for a 30% estimated glomerular filtration rate decline from the baseline.
Figure S2| Results of a Kaplan–Meier analysis for an estimated glomerular filtration rate <60 mL/min/1.73 m2.
Figure S3| Results of a Kaplan–Meier analysis for a 40% estimated glomerular filtration rate decline from the baseline, stratified by cardiovascular autonomic neuropathy and diabetic peripheral sensory neuropathy.
ACKNOWLEDGMENTS
The authors thank students of the Department of Public Health and Epidemiology, Meiji Pharmaceutical University, and staff in the clinic of the Institute for Medical Science, Asahi Life Foundation, for helping with data collection.
J Diabetes Investig 2022; 13: 102–111
REFERENCES
- 1. Kastarinen M, Juutilainen A, Kastarinen H, et al. Risk factors for end‐stage renal disease in a community‐based population: 26‐year follow‐up of 25,821 men and women in eastern Finland. J Intern Med 2010; 267: 612–620. [DOI] [PubMed] [Google Scholar]
- 2. Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983; 32(Suppl 2): 64–78. [DOI] [PubMed] [Google Scholar]
- 3. MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004; 27: 195–200. [DOI] [PubMed] [Google Scholar]
- 4. Kramer HJ, Nguyen QD, Curhan G, et al. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 289: 3273–3277. [DOI] [PubMed] [Google Scholar]
- 5. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet 2010; 375: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Penno G, Solini A, Orsi E, et al. Non‐albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicentre study. Diabetologia 2018; 61: 2277–2289. [DOI] [PubMed] [Google Scholar]
- 7. Iseki K, Ikemiya Y, Iseki C, et al. Proteinuria and the risk of developing end‐stage renal disease. Kidney Int 2003; 63: 1468–1474. [DOI] [PubMed] [Google Scholar]
- 8. Low PA, Benrud‐Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population‐based study. Diabetes Care 2004; 27: 2942–2947. [DOI] [PubMed] [Google Scholar]
- 9. Mendivil CO, Kattah W, Orduz A, et al. Neuropad for the detection of cardiovascular autonomic neuropathy in patients with type 2 diabetes. J Diabetes Complications 2016; 30: 93–98. [DOI] [PubMed] [Google Scholar]
- 10. Maser RE, Mitchell BD, Vinik AI, et al. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta‐analysis. Diabetes Care 2003; 26: 1895–1901. [DOI] [PubMed] [Google Scholar]
- 11. Pop‐Busui R, Evans GW, Gerstein HC, et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010; 33: 1578–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gelber DA, Pfeifer M, Dawson B, et al. Cardiovascular autonomic nervous system tests: determination of normative values and effect of confounding variables. J Auton Nerv Syst 1997; 62: 40–44. [DOI] [PubMed] [Google Scholar]
- 13. Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta‐analysis. Neurosci Biobehav Rev 2016; 64: 288–310. [DOI] [PubMed] [Google Scholar]
- 14. Schumer MP, Joyner SA, Pfeifer MA. Cardiovascular autonomic neuropathy testing in patients with diabetes. Diabetes Spectrum 1998; 11: 227. [Google Scholar]
- 15. Ewing DJ, Martyn CN, Young RJ, et al. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985; 8: 491–498. [DOI] [PubMed] [Google Scholar]
- 16. Yun J‐S, Ahn Y‐B, Song K‐H, et al. The association between abnormal heart rate variability and new onset of chronic kidney disease in patients with type 2 diabetes: a ten‐year follow‐up study. Diabetes Res Clin Pract 2015; 108: 31–37. [DOI] [PubMed] [Google Scholar]
- 17. Tahrani AA, Dubb K, Raymond NT, et al. Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: a cohort study. Diabetologia 2014; 57: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 18. Sonoda R, Tanaka K, Kikuchi T, et al. C‐peptide level in fasting plasma and pooled urine predicts HbA1c after hospitalization in patients with type 2 diabetes mellitus. PLoS One 2016; 11: e0147303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 20. Meyer NL, Mercer BM, Friedman SA, et al. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol 1994; 170: 137–141. [DOI] [PubMed] [Google Scholar]
- 21. Yasuda H, Sanada M, Kitada K, et al. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract 2007; 77(Suppl 1): S178–183. [DOI] [PubMed] [Google Scholar]
- 22. Agelink MW, Malessa R, Baumann B, et al. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin Auton Res 2001; 11: 99–108. [DOI] [PubMed] [Google Scholar]
- 23. Bhatraju PK, Zelnick LR, Shlipak M, et al. Association of soluble TNFR‐1 concentrations with long‐term decline in kidney function: the multi‐ethnic study of atherosclerosis. J Am Soc Nephrol 2018; 29: 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka K, Hara S, Hattori M, et al. Role of elevated serum uric acid levels at the onset of overt nephropathy in the risk for renal function decline in patients with type 2 diabetes. J Diabetes Investig 2015; 6: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prince CT, Secrest AM, Mackey RH, et al. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 2010; 33: 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valensi P, Pariès J, Attali JR. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications–the French multicenter study. Metabolism 2003; 52: 815–820. [DOI] [PubMed] [Google Scholar]
- 27. Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006; 55: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 28. Ravid M, Savin H, Jutrin I, et al. Long‐term stabilizing effect of angiotensin‐converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med 1993; 118: 577–581. [DOI] [PubMed] [Google Scholar]
- 29. Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16: 3027–3037. [DOI] [PubMed] [Google Scholar]
- 30. Yoshinari M, Wakisaka M, Nakamura U, et al. Orthostatic hypertension in patients with type 2 diabetes. Diabetes Care 2001; 24: 1783–1786. [DOI] [PubMed] [Google Scholar]
- 31. Bigger JT, Fleiss JL, Steinman RC, et al. RR variability in healthy, middle‐aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation 1995; 91: 1936–1943. [DOI] [PubMed] [Google Scholar]
- 32. Pfeifer MA, Cook D, Brodsky J, et al. Quantitative evaluation of cardiac parasympathetic activity in normal and diabetic man. Diabetes 1982; 31: 339–345. [DOI] [PubMed] [Google Scholar]
- 33. Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes 2014; 5: 17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ewing DJ, Borsey DQ, Bellavere F, et al. Cardiac autonomic neuropathy in diabetes: comparison of measures of R‐R interval variation. Diabetologia 1981; 21: 18–24. [DOI] [PubMed] [Google Scholar]
- 35. Spallone V, Gambardella S, Maiello MR, et al. Relationship between autonomic neuropathy, 24‐h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care 1994; 17: 578–584. [DOI] [PubMed] [Google Scholar]
- 36. Wheelock KM, Jaiswal M, Martin CL, et al. Cardiovascular autonomic neuropathy associates with nephropathy lesions in American Indians with type 2 diabetes. J Diabetes Complications 2016; 30: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malik S, Winney RJ, Ewing DJ. Chronic renal failure and cardiovascular autonomic function. Nephron 1986; 43: 191–195. [DOI] [PubMed] [Google Scholar]
- 38. Rubinger D, Backenroth R, Sapoznikov D. Restoration of baroreflex function in patients with end‐stage renal disease after renal transplantation. Nephrol Dial Transplant 2009; 24: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 39. Salman IM. Cardiovascular autonomic dysfunction in chronic kidney disease: a comprehensive review. Curr Hypertens Rep 2015; 17: 59. [DOI] [PubMed] [Google Scholar]
- 40. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 41. Gæde P, Vedel P, Parving H‐H, et al. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999; 353: 617–622. [DOI] [PubMed] [Google Scholar]
- 42. Hotta N, Akanuma Y, Kawamori R, et al. Long‐term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3‐year, multicenter, comparative Aldose Reductase Inhibitor‐Diabetes Complications Trial. Diabetes Care 2006; 29: 1538–1544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1| Results of a Kaplan–Meier analysis for a 30% estimated glomerular filtration rate decline from the baseline.
Figure S2| Results of a Kaplan–Meier analysis for an estimated glomerular filtration rate <60 mL/min/1.73 m2.
Figure S3| Results of a Kaplan–Meier analysis for a 40% estimated glomerular filtration rate decline from the baseline, stratified by cardiovascular autonomic neuropathy and diabetic peripheral sensory neuropathy.


