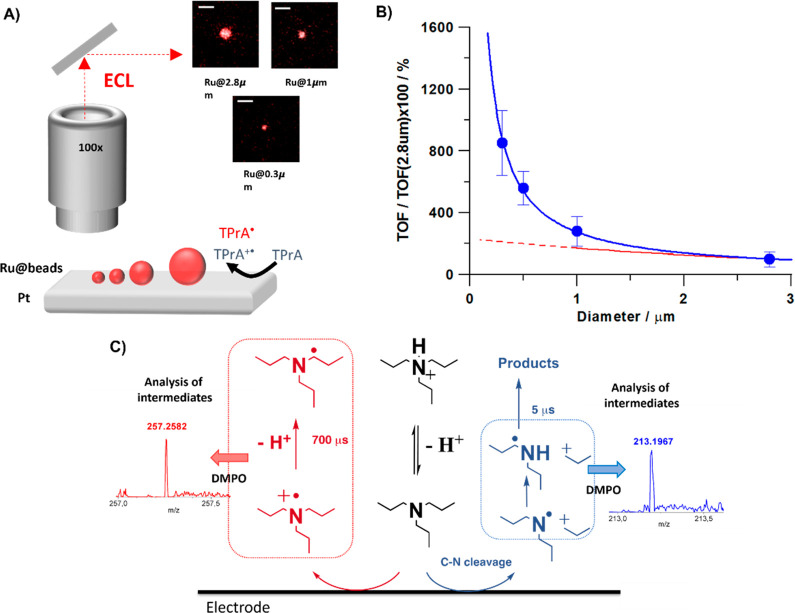
Figure 5.
(A) Schematic representation of the surface generation-bead emission experiment on beads with different sizes where Ru@2.8 μm, Ru@1 μm, and Ru@0.3 μm are ECL images of magnetic beads labeled with [Ru(bpy)3]2+ with a diameter of 2.8, 1, and 0.3 μm, respectively. Magnification ×100; scale bar 5 μm; potential applied, 1.4 V (vs Ag/AgCl, 3 M KCl); acquisition time, 0.5 s. (B) Turnover frequency (TOF) as a function of bead size. (C) Schematic representation of the proposed parallel pathways for the tri-n-propylamine (TPrA) oxidation at the electrode where TPrA•+ and TPrA• are generated (red pathway) and where dipropylamine radical (DPrA) is generated (blue pathway). The scheme reaction is supported by spin-trapping experiments with 5,5-dimethyl-pyrroline N-oxide (DMPO), which stabilized the radicals and allowed identification by mass spectrometry analysis (MS) and electron paramagnetic resonance (EPR). The inset shows the MS analysis for the possible adducts DMPO-TPrA and DMPO-DPrA. Reprinted by permission from Springer Nature Limited: Nature Communications, Zanut, A.; Fiorani, A.; Canola, S.; Saito, T.; Ziebart, N.; Rapino, S.; Rebeccani, S.; Barbon, A.; Irie, T.; Josel, H.-P.; et al. Insights into the Mechanism of Coreactant Electrochemiluminescence Facilitating Enhanced Bioanalytical Performance. Nat. Commun.2020, 11 (1), 2668, DOI: 10.1038/s41467-020-16476-2 (ref (4)). Copyright 2020 Springer Nature Limited.