Figure 4.
Clinical features, transcriptional studies, and identification of mutation in DMD PF
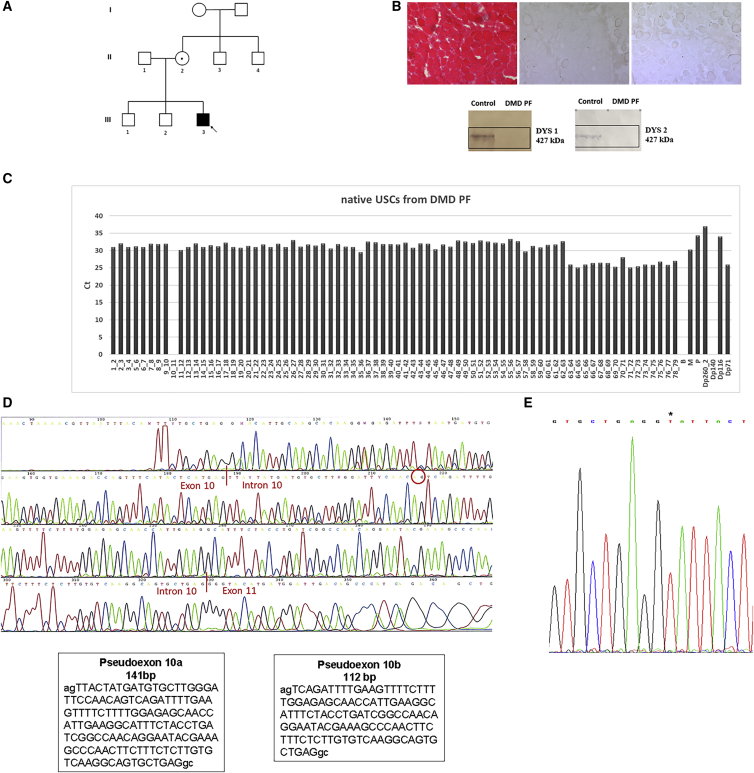
(A) Pedigree of DMD individual PF’s family. The index individual (III3, arrow) has a DMD diagnosis and carries the c.1149+250C>T mutation in intron 10 of the DMD gene (D, right). The individual’s mother (II2) is a carrier of the DMD mutation.
(B) Histological analysis with hematoxylin and eosin (H&E) (left part) and immunostaining with the dystrophin antibody to rod-domain and C-terminal regions (right part) of muscle sections from DMD PF. Dystrophic changes (variation in fiber size, fibrosis, inflammatory infiltrate) are observed with H&E and only few revertant fibers are dystrophin positive. Western blot analysis (lower part) shows the complete absence of the dystrophin protein in the DMD PF muscle.
(C) Expression of dystrophin transcript in native USCs from DMD individual PF. FluiDMD shows the absence of amplification of the exon-exon junction 10–11 of the dystrophin transcript. Ct, cycle threshold.
(D) Sequencing of the dystrophin transcript of the DMD individual. The region spanning exons 10–12 was amplified, and PCR products were sequenced. Two transcripts spliced between exon 10 and exon 11 were found, the first one of 141 bp (pseudo-exon 10a, left box) and the second one of 112 bp (pseudo-exon 10a, right box); the red circle highlights the acceptor site of the shorter pseudo-exon 10b.
(E) Sequencing of intron 10 identified the pathogenic mutation c.1149+250C>T (∗) generating a donor splice site.