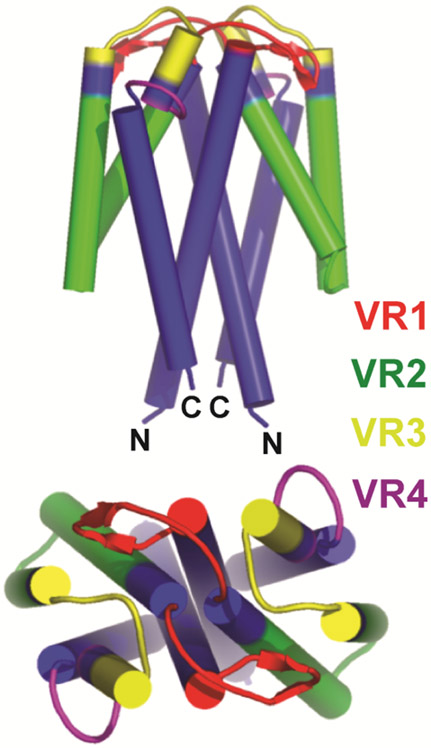
Figure 4.
Schematic representation of structure of a Vsp lipoprotein dimer of B. turicatae. The four major alpha-helical chains in each monomer are depicted by cylinders. The first and fourth alpha-helix (blue) in each Vsp are relatively conserved in sequence between different Vsp proteins of a given species (Dai et al., 2006; Restrepo et al., 1992). The second and third alpha-helical chain and the loops between all the chains are variable in sequence and are divided into variable regions (VR) 1 (red), 2 (green), 3 (yellow), and 4 (purple). The greatest variability is at the top or dome of the protein. Adapted from Lawson et al. (Lawson et al., 2006).