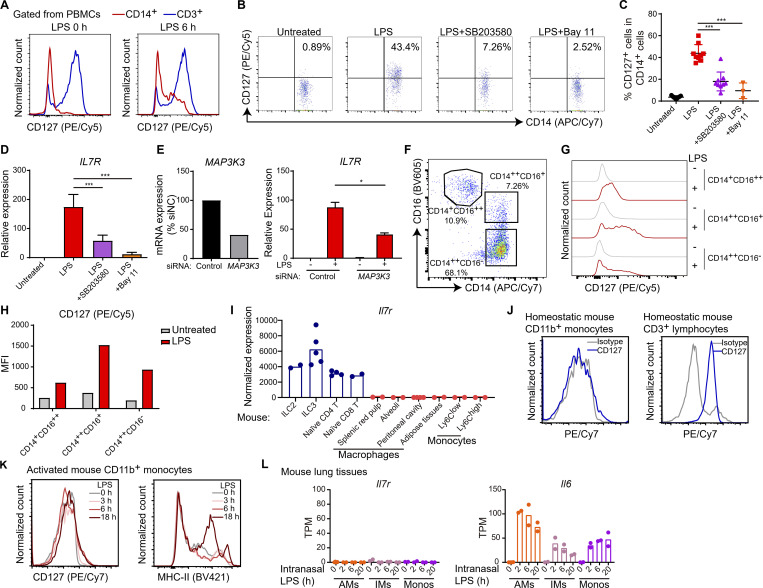
Figure S2.
Elicited CD127 expression represents a common but unique feature for activated human monocytes and is induced by TLR signaling. (A) PBMCs from healthy donors were treated with or without 6-h LPS stimulation (10 ng/ml), and CD127 expression in CD3+ T cells and CD14+ monocytes was analyzed by FACS in each condition. Representative FACS distributions are shown from three independent experiments. (B and C) PBMCs form healthy donors were pretreated with DMSO or 10 µM SB203580 or 10 µM Bay 11-7082 (Bay 11) for 30 min and subsequently stimulated with or without 10 ng/ml LPS for 6 h as indicated. The protein levels of CD127 were measured by FACS and are shown as representative FACS distribution (B) and cumulative percentages (C) in CD14+ monocytes. ***, P < 0.001 by unpaired t test. Each data point represents an independent experiment. Data are shown as the mean ± SD of multiple independent experiments as listed, respectively: untreated n = 9, LPS n = 9, SB203580 n = 9, Bay 11-7082 n = 3. (D) CD14+ selected monocytes from healthy donors were pretreated with DMSO or 10 µM SB203580 or 10 µM Bay 11-7082 (Bay 11) for 30 min and subsequently stimulated with or without 10 ng/ml LPS for 6 h as indicated. mRNA of IL7R was measured by qPCR. The relative expression was normalized to internal control (GAPDH) and expressed relative to the untreated sample. ***, P < 0.001 by unpaired t test. Data are shown as the mean ± SD of multiple independent experiments as listed, respectively: untreated n = 9, LPS n = 9, SB203580 n = 6, Bay 11-7082 n = 3. (E) CD14+ monocytes were transfected with negative control or MAP3K3-specific siRNAs. 2 d after transfection, cells were stimulated with LPS (10 ng/ml) for 3 h. Knockdown efficiency of MAP3K3 was examined, and mRNA induction of IL7R by LPS stimulation in siControl and siMAP3K3 transfected cells was measured by qPCR. Relative expression was normalized to internal control (GAPDH) and expressed relative to LPS untreated siControl sample. *, P < 0.05 by paired t test. IL7R expression data are shown as the mean ± SD of three independent experiments. (F–H) Three human monocyte populations were gated by CD14 and CD16 expression in FACS analysis of healthy donor PBMCs as shown in F. CD127 expression in three human monocyte populations was analyzed in conditions with or without LPS stimulation for 6 h. Representative FACS distribution (G) and mean fluorescence intensity (MFI; H) for CD127 expression are shown from three independent experiments. (I) The normalized Il7r expression level assessed by RNA-seq was obtained from the ImmGen database, and expression levels in multiple mouse immune cell types are shown, including ILC2 and ILC3 in small intestines, naive CD4 and CD8 T cells in spleens, macrophages across different tissues, and Ly6C delimited blood monocyte populations. Each data point represents each individual replicate sample included in the ImmGen database. (J and K) Mouse blood cells (red blood cells lysed) were treated with or without 100 ng/ml LPS for different time points as indicated. In the homeostatic condition, CD127 expression in mouse CD11b+ monocytes was analyzed by FACS, and expression in CD3+ lymphoid cells served as a positive control (J). Upon LPS stimulation, the expression of CD127 and MHC-II on mouse CD11b+ monocytes was analyzed by FACS (K). (L) The published RNA-seq datasets were generated in macrophages and monocytes isolated from mouse lung tissues, where mice were intranasally treated with 10 µg LPS per mouse for different time points. The expression levels (gene-specific transcripts per million total transcripts [TPMs]) of Il7r and Il6 were assessed and are shown across alveolar macrophages (AMs), interstitial macrophages (IMs), and tissue monocytes (Monos). Each data point represents each independent biological replicate in the original datasets. Independent experiments in A–H were performed with cells from one healthy donor for each experiment.