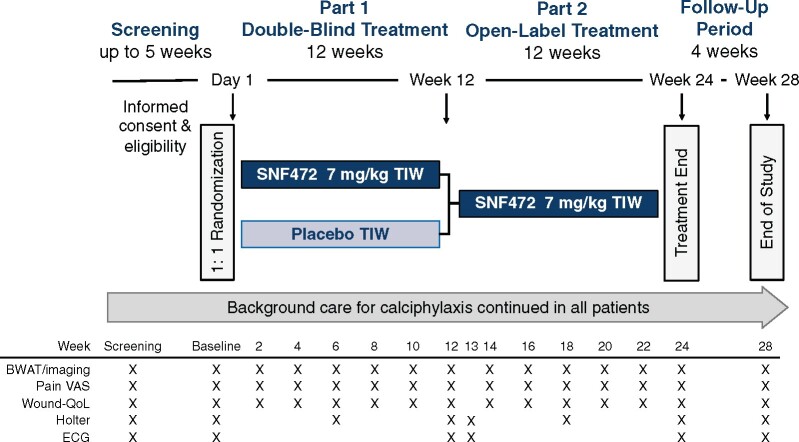
FIGURE 1:
Study design. After a screening period of up to 5 weeks, eligible patients will be randomized 1:1 to receive an infusion of SNF472 or placebo TIW, during haemodialysis. Double-blind study treatment will be administered for 12 weeks, followed by open-label SNF472 treatment for an additional 12 weeks, and a 4-week safety follow-up period. Background care, including pain medications and/or STS, will be stabilized during screening and no changes to the background care regimen will be made after randomization unless medically indicated in the opinion of the Investigator.