Figure 4.
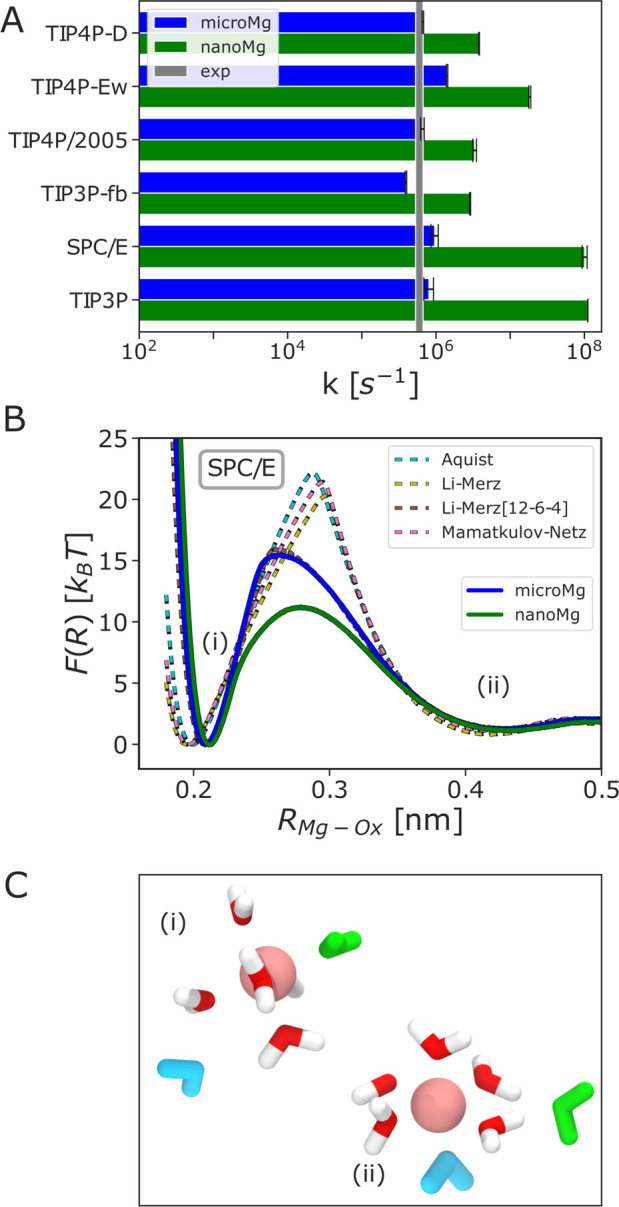
Water exchange in the first hydration shell of Mg2+. (A) Kinetic rate coefficients k (eq S3) of microMg (blue) and nanoMg (green) of different water models (SPC/E,46 TIP3P-fb,47 TIP4P/2005,48 TIP4P-Ew,49 and TIP4P-D50) and for TIP3P from the literature.26 The gray vertical line indicates the experimental values73,74 (Table 4). (B) One-dimensional free energy profiles as a function of the distance between Mg2+ and the leaving water molecule RMg–Ox for different force fields in combination with SPC/E water. (C) The snapshots show representative conformations in the two stable states: (i) Before exchange: Leaving water (shown in green) is part of the first and entering water (shown in blue) is part of the second hydration shell. (ii) After exchange: Leaving water is in the second hydration shell and the entering water molecule filled the void in the first hydration shell. The snapshots were taken using microMg(SPC/E).
