Abstract
Understanding the developmental timing of stress exposure may help inform mechanisms underlying how stress “gets under the skin” and influences the stress response system, including the HPA axis and its end-product cortisol. Early adversity may be particularly detrimental; however, it is difficult to disentangle the timing of adversity from its cumulative burden because there is typically high continuity between early and later adversity. Moreover, context and the different stressors inherent in various contexts may interact with stress exposure to influence psychophysiological functioning. To address this issue, we examined adolescents who had been reared in institutions and suffered neglect or social deprivation ranging from approximately six months to several years of life prior to adoption into U.S. homes. We focused on the stress hormone cortisol because it can reflect continued regulatory problems in youth, even years after youth transition to typical homes. We examined cortisol morning levels and diurnal rhythms across multiple contexts (home, school, lab) on 5 separate days in 41 post-institutionalized youth and 78 comparison youth. Employing hierarchical linear modeling, we found that when assessed in the lab, post-institutionalized (PI) youth displayed lower morning cortisol levels and flatter diurnal slopes than the control youth. Yet at home, PI youth displayed higher morning cortisol levels than the control youth. In addition to group effects, we also examined severity of early adversity and found that PI kids who had endured the most severe early adversity displayed lower home cortisol levels than controls. No significant predictors of diurnal cortisol on school days were identified. These data fit with the notion that the HPA axis is impacted by early adversity, even years after adoption, and with emerging theories that postulate that stress physiology calibrates within youth to help them adapt to their context. In the case of severe early adversity, the cost of such adaptation may not be desirable. It also highlights the important role of context when assessing HPA axis activity, particularly in post-institutionalized youth.
Keywords: Adolescence, Arousal and Regulatory Systems, Cortisol, Post-Institutionalized Youth, Adversity
1.0. Introduction
Exposure to physical, social, and psychosocial stressors is a leading risk factor for mental and physical health problems throughout the entire lifespan (Yen & Syme, 1999), with profound impact when stress exposure occurs within the early years of a child’s life (Boyce, 2009; Shonkoff, 2010). There is increasing awareness that the impact of stress is mediated in part through stress responsive physiology (Lupien et al., 2006). Emerging theories suggest that the impact of adversity is complex and, broadly speaking, both hyper- and hypo-arousal of the stress system can stem from adversity (Ellis, Del Giudice, & Shirtcliff, 2012; Hostinar & Gunnar, 2013; Smith & Pollak, in press). Physiological changes are adaptive, but not necessarily desirable – a frequent cost of adaptation includes health problems that manifest as short-term adjustment and long-term trade-offs (Korte, Koolhaas, Wingfield, & McEwen, 2005; McEwen & Wingfield, 2003). The present study focuses on cortisol as a stress system measure because this end-product of the hypothalamic-pituitary-adrenal (HPA) axis (a) is responsive to social context of both short- and long-term duration (Miller, Chen, & Zhou, 2007), (b) has a relatively high threshold for activation compared to other stress systems (i.e., the autonomic nervous system), so it likely reflects only the most salient of stressors rather than more minor stressors such as being stuck in traffic (Sapolsky, Romero, & Munck, 2000), and (c) is capable of changing gene expression, such that the impact of HPA functioning is likely to persist for a substantial period of time (De Kloet, 2004). This study focuses on a unique population of adolescents who experienced social and emotional neglect within the first years of life in order to discern timing of adversity. Framed within theories of hyper- and hypo-arousal and based on studies that find long-term effects on the HPA axis (Essex et al., 2011), we anticipated that cortisol functioning, in this case diurnal rhythms and variation across contexts, would continue to reflect early adversity even years after youth transitioned to enriched homes and the extreme adversity terminated.
1.1. Theories and Empirical Findings for HPA Hyper-Arousal After Early Adversity
Early adversity may sensitize the HPA axis to stress, i.e., HPA hyper-arousal (Struber, Struber, & Roth, 2014). For example, Essex and colleagues (2002) found that preschoolers with concurrent stress exposure had elevated cortisol levels, especially if they had been exposed to high stress earlier in life. Others found hyper-arousal within pre-adolescents and adolescents exposed to early adversity and maltreatment both in terms of reactive (Harkness et al., 2011) and diurnal cortisol levels (O’Connor et al., 2005). Some research has found effects of early adversity are stable and persistent, as when hyper-arousal is found within adults with prior history of child maltreatment (diurnal: Nicolson et al., 2010; reactive: Carpenter et al., 2009; Carpenter et al., 2011).
Theoretical models for hyper-arousal are largely functional, emphasizing that the HPA axis serves a purpose. HPA hyper-arousal may indicate an exaggerated emotional response to a stressor (Jackson et al., 2006) and suggests the individual lacked the internal resources to cope sufficiently with a stressor and minimize HPA arousal. This functional view acknowledges that some responsivity is appropriate (Dickerson and Kemeny, 2004), but may become problematic if extreme or prolonged (Bosch et al., 2009; Sjogren, Leanderson, & Kristenson, 2006) or if the situation is excessively uncontrollable or threatening. A parallel functional explanation emphasizes that cortisol is sensitive to supportive aspects of the environment (Shirtcliff et al., 2014; Shirtcliff et al., 2017), such as positive parenting or caregiving quality (Nachmias et al., 1996; Gunnar et al. 2015; Hostinar, Johnson, & Gunnar, 2015). The potential for caregiving quality to buffer a stress response is eliminated if support is not provided by an attentive caregiver (Hostinar & Gunnar, 2013) or if the individual is less sensitive to stress buffering (Doom et al., 2015). This may be the case with early neglect, which involves disruptions or absence of a nurturing caregiver (Hussey, Chang, & Kotch, 2006). Functional theories extend beyond acute stressors as physiological stress mediators are utilized in a variety of day-to-day situations when internal (i.e., neural) resources or external (i.e., caregiver) secure base can enhance the individual’s ability to cope with surmountable challenges.
1.2. Theories and Empirical Findings for HPA Hypo-Arousal after Early Adversity
One concern with these functional theories is that HPA hypo-arousal is also linked with adversity, and increasingly is recognized as a form of risk (Shirtcliff et al., 2009; Susman, 2006). Several studies on children placed in foster care reveal blunted or low cortisol within the most stress-exposed children (Fisher & Stoolmiller, 2008; Fisher et al., 2007), which may normalize with improved environmental conditions (Fisher, Van Ryzin, & Gunnar, 2011). Child maltreatment may also be linked with an attenuated stress response (MacMillan et al., 2009) or blunted daily levels (Fernald, Burke, & Gunnar, 2008), even years later during adulthood (reactive: Carpenter et al., 2011; Carpenter et al., 2007; diurnal: Kuras et al., 2017).
Theoretical explanations for HPA hypo-arousal emphasize development and time-course. Miller and colleagues (2007) describe how hypo-arousal unfolds depending on the time-course following a traumatic event or extreme stressor. More specifically, while hyper-arousal may have manifested initially, over time, the stress response is so excessive or prolonged that mounting an elevated response to environmental threat too easily damages the brain and body. At the adrenal level, the child’s threshold for mounting a stress response increases over time as they show signs of habituation, even to extremely chaotic environments (Wust et al., 2005). When confronted by normative social challenges, the individual is nonresponsive, even if a stress response had been appropriate. Given that it is adaptive to be responsive to stress and to competently terminate a stress response, individuals with down-regulated HPA axis activity may be paradoxically at heightened risk for stress-related diseases (Wismer Fries et al., 2005).
1.3. Post-Institutionalized Youth Experience Extreme Early Adversity
Post-institutionalized youth experience profound adversity within the first few years of life. Variations in the degree of neglectfulness both across and within orphanages are present, yet conditions have been characterized as ranging from poor to appalling (Human Rights Watch, 1998) and were especially poor at the time in which the youth in the current study had been in the orphanage setting, evidenced by poor health, growth failure, and developmental delays (Johnson, 2000; Miller et al., 2007). Furthermore, the child’s needs for a stable, consistent relationship were also unlikely to be met within the first few years of life (Gunnar, Bruce, & Grotevant, 2000), which often results in later difficulties with attachment, bonding and social or emotional adjustment (Loman et al., 2013; Rutter & O’Connor, 2004; Wiik et al., 2011).
Despite the precision afforded by knowing the date at which adversity is terminated, prior research on post-institutionalized youth finds both hyper-arousal (basal/ diurnal: Fries, Shirtcliff, & Pollak, 2008; Gunnar et al., 2001; Johnson et al., 2011) and hypo-arousal of the HPA axis (reactive: Hostinar, Johnson, & Gunnar, 2015; Koss et al., 2016; McLaughlin et al., 2015; Quevedo et al., 2012; basal/ diurnal: Koss et al., 2014; Koss et al., 2016; Kroupina et al., 2012). Moreover, when exposed to a psychosocial stressor both hyper- and hypo-cortisol reactivity were apparent in post-institutionalized youth; however, the most severely affected youth were most similar to comparison children (Gunnar et al., 2009), suggesting that these seemingly divergent patterns of findings are not due to study-specific nuances, but may reflect a diversity of underlying effects of adversity on HPA functioning.
1.4. The Effect of Context on Diurnal Cortisol in Post-Institutional Youth
An important factor often left unexamined or held constant within diurnal cortisol research is context. Novel and unpredictable contexts (Gunnar et al., 2000) and social salience (Stroud, Salavey, & Epel, 2002) have been identified as important moderators of cortisol (e.g., entering a new peer group setting; Bruce, Davis, & Gunnar, 2002; Gunnar, Tout, de Haan, Pierce, & Stansbury, 1997; Quas, Murowchick, Bensadoun, & Boyce, 2002; Sanchez-Martin et al., 2001; Tout, de Haan, Campbell, & Gunnar, 1998). The diurnal rhythm of cortisol is altered in response to the anticipation or experience of stressors, especially social stressors, during the day (Adam, 2002; Smyth et al., 1998; Smyth et al., 1997). Therefore, it is plausible that interacting with peers at school, or novelty introduced by the laboratory setting, temporarily alters children’s diurnal rhythms. It is also possible that these alterations may be most prominent in post-institutionalized (PI) youth given that child abuse and neglect often negatively impact children’s social and emotional adjustment and thus their ability to adequate handle these types of stressors (Cicchetti & Lynch, 1995; Cicchetti & Walker, 2001; Pollak, Cicchetti, & Klorman, 1998).
1.5. The Current Study and Hypotheses
We hypothesize that PI adolescents will show altered HPA functioning compared to comparably aged youth; due to divergence of past results, we were agnostic as to whether hyper- or hypo-arousal of the HPA axis would be discovered. We hypothesize that the context of sample collection may impact whether hyper- or hypo-arousal is observed, with the laboratory context of the present study acting as both a novel and social setting, the school setting to operating as a familiar environment but may still operate as a social challenge for some PI youth who often experience peer rejection and victimization (e.g., Pitula et al., 2014; Raaska et al., 2013), and the home setting functioning as a non-stressful comparison day; however, PI youth may still exhibit altered diurnal cortisol due to the long-lasting effects of abuse and neglect. Additionally, there is a range of stress and adversity experienced across the control and post-institutionalized youth, as well as a range of stress and institutionalization experiences within the group of PI youth; therefore, we examine whether stress exposure or measures related to institutionalization (i.e., severity of physical/ social neglect, amount of time in the institution, number of different living situations) impact cortisol and look for differences between the PI youth and controls, as well as differences in the severity of adverse experiences within the group of PI youth. We hypothesize that the experience of being institutionalized in the first years of life would exert a unique impact on the HPA axis, but this would partially overlap mechanistically with other stress exposure. Additionally, we explore whether stress exposure and institutionalization variables exert a unique effect on cortisol, independent of Group status. Given the exploratory nature of these analyses, results should be interpreted as representing a shared construct and not unique, independent effects.
2.0. Method
A total of 119 adolescents participated in this study, aged 9–14 years (M=11.15, SD=1.7), including 58 males (48%) and 61 females (62%). Most youth were Caucasian (N=72, 61%), but there was representation of African American (N=16, 13%), other race (N=16, 13%), Asian (N=6, 5%), mixed (N=5, 4%), and Hispanic youth (N=4, 3%; see Table 1 for sample demographics). Youth with signs of fetal alcohol exposure or fetal alcohol syndrome were excluded by a medical geneticist who reviewed participants’ facial photographs for (1) distance between the endocanthion and exocanthion landmarks, (2) philtrum smoothness, and (3) upper lip thinness (Astley et al. 2002).
Table 1.
Sample Demographic Variables in Comparison and Post-Institutionalized Youth
| Comparison | PI | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| (N = 78) | (N = 41) | |||||
|
| ||||||
| M | SD | M | SD | F | ||
| Age | 11.21 | 1.71 | 11.05 | 1.70 | 0.003 | |
| SES | 46.40 | 14.12 | 52.88 | 8.70 | 8.702** | |
| Tanner Stage | 1.68 | 1.27 | 1.31 | 1.12 | 1.618 | |
|
| ||||||
| n | % | n | % | χ 2 | ||
|
| ||||||
| Sex | 0.586 | |||||
|
| ||||||
| Female | 38 | 49 | 23 | 56 | ||
| Male | 40 | 51 | 18 | 44 | ||
|
| ||||||
| Race | 47.228*** | |||||
|
| ||||||
| White Caucasian | 51 | 65 | 23 | 51 | ||
| African American | 16 | 21 | 0 | 0 | ||
| Other Race | 0 | 0 | 16 | 39 | ||
| Asian | 2 | 3 | 4 | 10 | ||
| Mixed | 5 | 6 | 0 | 0 | ||
| Hispanic | 4 | 5 | 0 | 0 | ||
Note:
p<0.05
p<0.01
p<0.001.
To understand the effects of an environment that changes drastically after early stress exposure, 41 participants (18 male; mean age = 11.1 years, SD = 1.7) who were internationally adopted from institutions for orphaned or abandoned children after suffering neglect were recruited. These participants spent an average of 31.6 months in institutional care, with a range from 4 to 77 months (SD = 16.0 months). These children had environments that changed drastically after they were adopted into normative family settings, averaging 17 enriched early experiences after adoption out of a possible 22 experience items (e.g., trips to museums or concerts; participation in extracurricular activities outside of school, vacations to mountains or oceans or on an airplane, family vacations, or a scrapbook made about him/her). Post-institutionalized youth are contrasted with 78 comparison children (38 female; mean age = 11.2, SD = 1.7) with no history of prior institutionalization in an orphanage or other setting and recruited from the community.
2.1. Procedures
All procedures were approved by the Institutional Review Board of the University of Wisconsin-Madison. Parents and youth provided informed consent and assent, respectively. Activities lasted several hours, beginning at 9:00 AM on the lab day. Youth completed several novel laboratory activities which varied in intensity as a stressor, including arrival at an unfamiliar location (Klimes-Dougan et al., 2001), puberty exam, and an MRI (Eatough et al., 2009). After lunch, youth completed interviews and questionnaires and left in the early afternoon. Saliva sampling on the laboratory day provided extensive training opportunities on self-administered saliva collection for youth and parents. Participants were sent home with explicit training about saliva collection and materials for 2 subsequent home days and 2 school days, resulting in a total of 5 days of saliva collection and up to 32 samples per youth.
2.2. Measures
2.2.1. Salivary Cortisol.
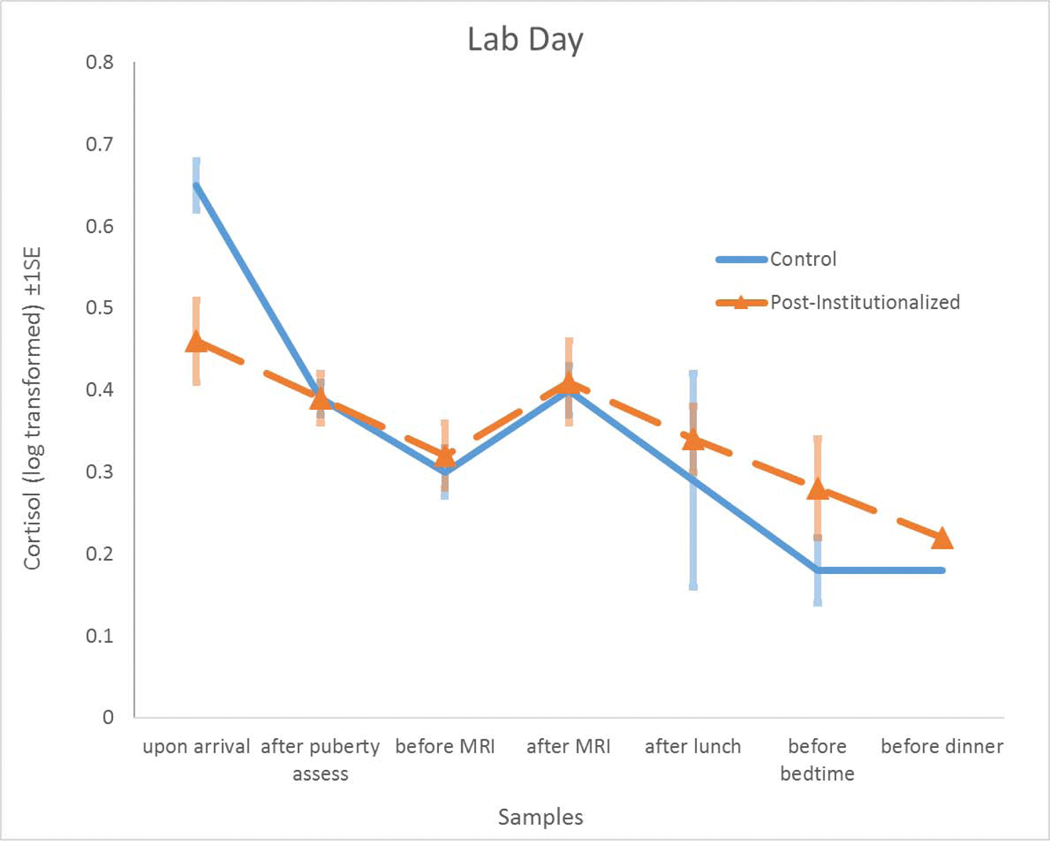
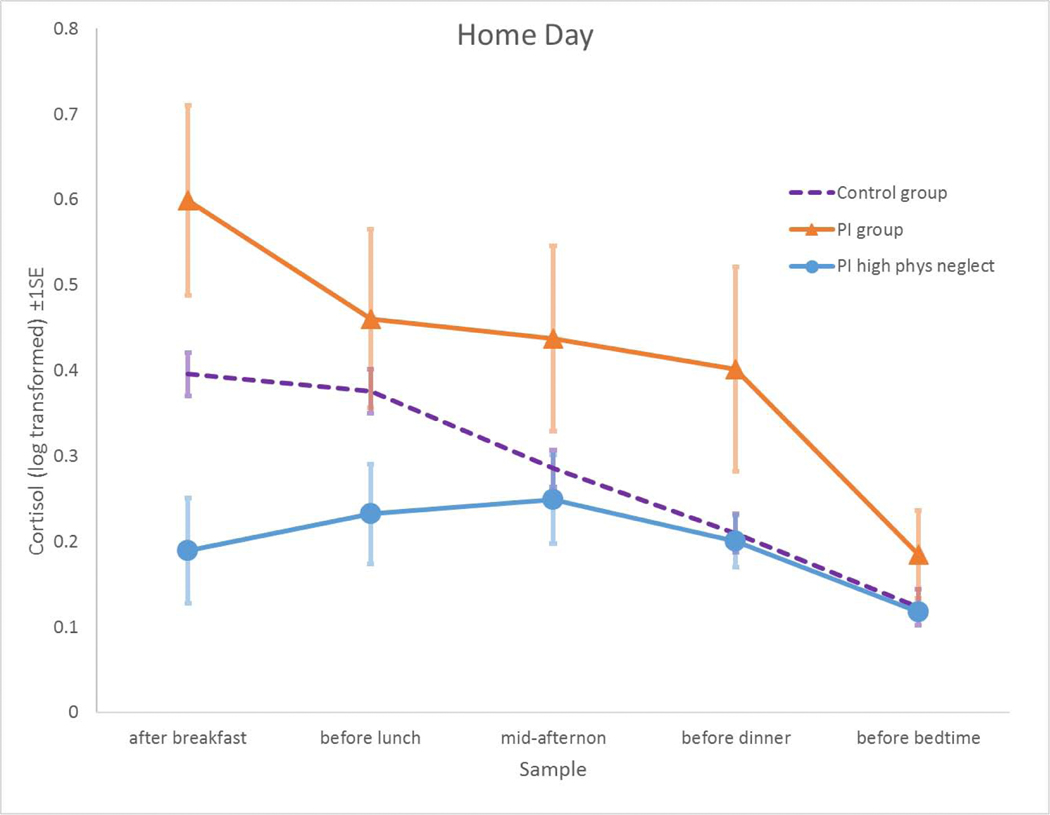
Each youth provided up to 32 saliva samples via passive drool, which were assayed for cortisol using a well-validated enzyme-immunoassay (www.salimetrics.com) and subsequently natural-log transformed to normalize the distribution. Manufacturer reported mean intra-assay and inter-assay coefficients of variation (CVs) are 4.6% and 6.0%, respectively. Youth completed a daily diary with each saliva sample. Saliva was immediately frozen at −80oC in the lab and collected (1) upon arrival (M = 9:45 AM, SD = 1:43), (2) after the puberty assessment (M = 10:29 AM, SD = 1:14), (3) before the MRI (M = 11:47 AM, SD = 1:08), (4) after the MRI (M = 12:14 PM, SD = 0:43), (5) after lunch (M = 1:39 PM, SD = 0:50), (6) after the interviews (M = 3:40 PM, SD = 1:01), (7) before dinner (M = 5:31 PM, SD = 1:23), and (8) at bedtime (M = 8:55 PM, SD = 1:34). On the lab-day, the final three samples (numbers 6 through 8) were collected at home after the child’s lab session was completed. Collection procedures for home and school days were identical to lab-day procedures with the exception that instructions were to (a) immediately freeze samples in home freezers when they got home from school; (b) record times of collection with additional verification of compliance by an electronic time-cap (www.aardexgroup.com); and (c) ship the samples frozen with freezer-brix overnight to the laboratory. After dropping the waking sample (M = 7:49 AM, SD = 1:31), self-administered sample collection times for home and school days largely paralleled laboratory-times: (1) mid-morning at least an hour after breakfast (M = 9:48 AM, SD = 1:13), (2) before lunch (M = 11:47 AM, SD = 1:08), (3) mid-afternoon (or after school on school-days) (M = 3:32 PM, SD = 1:10), (4) before dinner (M = 5:39 PM, SD = 1:14) and (5) at bedtime (M = 9:11 PM, SD = 1:46). On school-days, the mid-morning and before-lunch sample were collected at school and stored frozen with freezer-brix until transport to home freezers. Raw cortisol values (control mean = 0.136, S.D. = 0.191; PI mean = 0.144, S.D. = 0.156) were not normally distributed and required transformation. Average levels of log-transformed cortisol (+SE) across samples between days are visually shown in Figures 1 and 2. All cortisol outliers were windsorized to within 3 SD of the mean.
Figure 1.
As a group, Post-Institutionalized youth (Orange Lines) had slightly lower cortisol levels and a flatter slope on the lab day as compared to the Comparison Youth (Blue Line). Values plotted represent transformed cortisol levels (±standard error) of the sample means.
Figure 2.
Compared to control youth (dashed Purple Line), as a group, post-institutionalized youth (Orange Line) demonstrated hyper-arousal of HOMEDAY morning cortisol levels. However, HOMEDAY morning cortisol levels were lower for post-institutionalized youth who experienced the most severe neglect of physical needs (Blue Line; represents those who endorsed 3 on a 0–3 scale). The y-axis of Predicted Cortisol reflects Empirical Bayes estimates of cortisol levels extracted from the HLM analysis. Note: Similar, but slightly weaker, effects were identified upon conducting parallel analyses examining Neglect of Basic Social Needs.
2.2.2. Demographic Information.
We examined several demographic factors that can influence HPA functioning such as gender (male = 0, female = 1), age (in years), a composite of self-reported race, and socioeconomic status (Hollingshead 1975) (SES, M = 48.60 or middle- to -upper-middle class on average, SD = 12.90) (see Table 1). Within statistical models, youth were classified as Caucasian or Non-Caucasian to have adequate sample sizes within each group to analyze Child Race. To assess puberty, youth self-reported Tanner staging (Shirtcliff, Dahl, & Pollak 2009) and completed the Puberty Development Scale (PDS; Petersen et al. 1988). Next, experienced pediatric nurse practitioners conducted physical examinations. Assessments for girls involved palpation for breast development stage and visual examination of pubic hair. An orchidometer was used to measure testicular size in boys, along with visual inspection of pubic hair. Inter-observer reliability with nurse practitioners was excellent, (N = 10, K = 0.88). The PDS was converted to the Tanner Stage metrics (Shirtcliff, Dahl, & Pollak 2009) and then scores on the three puberty measures (Self- and Nurse-reported Tanner stage, Pubertal Development Scale) were averaged. Youth spanned the full Tanner stages of 1 – 5 (M = 2.55, SD = 1.23).
2.2.3. Youth Life Stress Interview.
To assess stress exposure, parents and youth separately completed the semi-structured Youth Life Stress Interview (LSI) with advanced graduate-level interviewers who received intensive training (Rudolph and Flynn 2007). Two types of stress exposure for the child were assessed: (1) Chronic Stress, and (2) Lifetime Adversity. Standardized probes were used to elicit objective information about stressful experiences across several life domains. A team of 3–6 coders then rated the interviews in a jury-like format following the interview and based on pre-determined anchors for stress ratings. Youth and parents were interviewed separately and responses were integrated within the coding session. For Chronic Stress, youth were questioned about specific domains (e.g., parent child stress, academic stress, marital/ family stress) and anchors were based on a scale of 1–5, where 2 is typical stress level and 5 is life-changing extreme stress. For Lifetime Adversity, a more general probe was issued to prompt youth to report on things that were very difficult for them, which were then followed up with probes about more specific situations (e.g., chronic illness of a family member, parental divorce, etc.). We used a ranking of 1–10 where 1 is no lifetime adversity and 10 is repeated, severe, stress exposure for this population. High reliability for the LSI has been achieved (Rudolph & Hammen 1999; Rudolph & Flynn 2007). To differentiate stress indices, Chronic Stress focused on the prior year whereas Lifetime Adversity focused on experiences across the youth’s lifetime excluding the prior year. Both control and post-institutionalized youth have scores which range in terms of severity of stress exposure (see Tables 2 and 3 for more information).
Table 2.
Stress Measures Capture a Range of Adversity within Comparison and Post-Institutionalized Youth.
| Comparison | PI | |||
|---|---|---|---|---|
|
| ||||
| (N = 78) | (N = 41) | |||
|
| ||||
| range | M (SD) | range | M (SD) | |
| Chronic Stress in the Past Year (LSI) | 1.21–3.43 | 2.26 (0.56) | 1.64–4.29 | 2.66 (0.64) |
| Lifetime Adversity (LSI) | 1.00–8.00 | 3.28 (2.07) | 2.00–10.00 | 4.63 (2.17) |
| Length of Time in Institution (in months) | - | - | 4.00–77.00 | 31.56 (15.99) |
| Number of Different Living Situations | - | - | 2.00–6.00 | 2.76 (0.97) |
| Institutional Conditions Index | - | - | 1.00–9.00 | 5.66 (2.54) |
| Neglect of Physical Needs | - | - | 0.00–3.00 | 1.27 (1.03) |
| Neglect of Basic Social Care | - | - | 0.00–3.00 | 1.44 (1.05) |
Table 3.
Correlations among Level 3 Predictors
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
|
| ||||||
| Level 3 Predictors | ||||||
| 1. Chronic Stress in the Past Year (LSI) | - | - | - | - | - | - |
| 2. Lifetime Adversity (LSI) | .454*** | - | - | - | - | - |
| 3. Length of Time in Institution | .255 | .412** | - | - | - | - |
| 4. Number of Different Living Situations | .282+ | .456** | .082 | - | - | - |
| 5. Institutional Conditions Index | .477** | .434** | .266+ | .382* | - | - |
| 6. Neglect of Physical Needs | .442** | .355* | .248 | .395* | .786*** | - |
| 7. Neglect of Basic Social Care | .483** | .484** | .182 | .476** | .743*** | .747*** |
p<0.10
p<0.05
p<0.01
p<0.001.
2.2.4. Post-Institutionalized Youth.
Families created by international adoption answered queries related to pre-adoption history; in the present study, we focus on five different variables: a) Length of Time in Institution: captures duration (in months) of neglect; b) Number of Different Living Situations: indexes stability of environment prior to adoption (Hanson et al. 2013); c) Institutional Conditions Index: captures a broad picture of children’s early living conditions (e.g., cleanliness, visible toys, responsiveness of caregiving, crowding) as rated by the adoptive parents based on their impressions of the institution in which their child resided prior to adoption on a 4-point scale: 0 (none) to 3 (severe); d) Neglect of Physical Needs: reflects the degree to which the parent suspects that child had adequate food, clothing, medical care, etc. while institutionalized on a 4-point scale: 0 (none) to 3 (severe); and e) Neglect of Basic Social Care: reflects the degree to which the parent suspects child lacked love, attention, cuddling, etc. while institutionalized on a 4-point scale: 0 (none) to 3 (severe). Given that these measures only apply to post-institutionalized youth, measures were constructed so that zero indicated no exposure to institutionalization and control youth were given scores of zero on all variables (see Table 2). Each variable was examined separately.
2.3. Analytic Strategy
Using HLM v.6 (Raudenbush, Bryk, & Congdon, 2004), the data was separated into three datasets to set up a three-level hierarchical linear model. We computed a three-level hierarchical linear model to deal with the inherent nesting of samples (Level 1: up to 32 samples per individual, total N=2,971), within days (Level 2: up to 5 days per individual, N=484), and within individuals (Level 3: N=118). A base model (below: also a parsimonious model) is used to illustrate multilevel modeling to test cortisol level of each sample i, nested in day j, nested in youth k, with cortisol level as the predicted outcome (LNCORTISOLijk). We then sought to explain change in cortisol across the day by including a time-since-waking (TSWijk) variable on Level 1 (quadratic and cubic functions of time-since-waking were also examined but were non-significant and therefore removed from subsequent analyses to avoid over-modeling). To account for an additional possibility that samples collected later in the day would be lower, we calculated a standardized residual score for the time of day (in hours) residualized for time since waking (CLOCKTIMEijk) so that higher scores would represent time of day independent of the diurnal slope. One participant was excluded from analyses due to missing data.
After modeling the within-person variability, day-to-day fluctuations, and examining the effect of control variables (see Descriptive Analyses section for more details), we explored the effect of Level 3 predictors. We were interested in disentangling whether the effect of Group (post-institutionalized vs. control) on the LAB/HOME/SCHOOLDAYjk effects was explained by prior stress exposure, the institutionalization measures, or if a curvilinear effect was identified; therefore after first examining the independent effect of Group, we then separately examined the effect of the stress exposure and institutionalization measures while retaining Group in the model (14 analyses). After this we then explored the possibility that prior experience may operate independent of group status by examining the linear main effects of stress exposure or institutionalization experience on LAB/HOME/SCHOOLDAY morning cortisol and slope (Group was not included in these models; 14 analyses).
2.3. Descriptive Analyses
Within an unrestricted HLM model including all five days of cortisol, 79.7% of the variance varied from moment-to-moment, less than 1% varied from one day to the next, χ2(365) = 603.3, p = 0.42, and 7.8% of the variance was stable within an individual across all samples, χ2(117) = 365.3, p < 0.001. After including our time-related variables, the Level 1 base model revealed that cortisol displayed the expected diurnal rhythm with samples declining across the day (linear: π1jk = −0.026, p < 0.001), and which was somewhat lower if samples were taken late (CLOCKTIME: π4jk = −0.012, p < 0.05). After modeling the diurnal rhythm, 25.35% of the variance in cortisol was specific to the moment of sample collection, 10.66% varied from one day to the next, χ2 (364) = 670.1, p < 0.001, and 63.99% was stable within an individual across all samples and operated like a trait, χ2 (117) = 328.98, p < 0.001.
Once the Level 1 base model was established, the predictors for cortisol level became outcomes of interest using a slopes-as-outcomes approach. Focusing on Level 2, predictors included time-varying measures which fluctuate across days but not within a single day such as the context measures. In the LABDAYjk model, the dichotomous variable LABDAYjk was included as a Level 2 predictor of morning cortisol levels (intercept) and diurnal rhythm (linear slope) and reflects diurnal cortisol level and slope on the days that participants visited the lab. The dichotomous variable SCHOOLDAYjk was included in the LABDAYjk model (on intercept and linear slope) as a control variable so that samples collected on the day they were in the lab were only compared to samples collected at home (not samples taken at school). Level 3 predictors were included on the intercept and slope (i.e., cortisol collected at home) and LABDAYjk intercept and slope (i.e., cortisol collected on the day that participants visited the lab). To examine differences between cortisol samples taken at school and those take at home, a similar model was examined, but the SCHOOLDAYjk was entered as the Level 2 predictor and LABDAYjk was included as a fixed control variable.
| Level 1 [within-individual, within-day] |
LNCORTISOLijk = π0ijk+ π 1ijk*TSWijk + π2ijk*CLOCKTIMEijk +eijk |
| Level 2 [day-level, within-individual] |
π 0jk=β00k+β01k*LABDAY02k+r0jk π 1jkTSW=β10k+β11k*LABDAY12k+r1jk π 2Jk=β20k+β21k*SCHOOLDAY22k [fixed] π 3jkTSW=β30k+β31k*SCHOOLDAY32k [fixed] π 4jkCLOCKTIME=β40k [fixed] |
| Level 3 [between-individuals] |
β00kLNCORTISOL intercept=γ000+u00k β01kLABDAY intercept=γ010 [fixed] β02kSCHOOLDAY intercept=γ020 [fixed] β10kLNCORTISOL slope=γ100+u10k β11kLABDAY slope=γ110 [fixed] β12kSCHOOLDAY slope=γ120 [fixed] β20kCLOCKTIME=γ200 [fixed] |
Note: Above is the depiction for the LABDAY cortisol model (controlling on SCHOOLDAY). A parallel model examining SCHOOLDAY (controlling on LABDAY) was also examined. LNCORTISOL variables reflect level of cortisol on days participants were at their homes.
Next we included demographic factors independently in the model as Level 3 predictors. Age, sex, pubertal status, and socioeconomic status did not significantly predict morning cortisol levels (intercepts) or diurnal rhythms (slopes) on home, school, or lab days, ps > 0.05, and therefore were excluded from subsequent analyses. A significant effect of child race was identified such that participants identifying as Caucasian had significantly flatter diurnal cortisol slopes than those identifying as non-Caucasian on school days (γ121 = 0.01, p = 0.031). Therefore, child race was included in any significant SCHOOLDAY models to examine if effects persisted.
3.0. Results
First, we examined a Level 2 base model examining the effect of context on cortisol levels and slopes. Results suggest that, when considering participants as a single group, there was no significant difference between cortisol collected at home and cortisol collected on lab days in terms of morning level or decline across the day (ps >.10). However, a trend-level finding was detected when examining school days, such that morning cortisol collected on school days was slightly lower than on home days (γ020 = −0.051, p < 0.10).
3.1. The Influence of Context and Institutionalization on LABDAY Diurnal Cortisol Rhythm
We found no Level 2 main effect of LABDAY on cortisol in terms of morning level or slope; however, upon including Group as a Level 3 predictor we found a trend-level effect on LABDAY cortisol morning level and a significant effect on LABDAY cortisol slope. This suggests that, when in the lab, PI youth had slightly lower morning cortisol levels and significantly flatter diurnal rhythms than controls. The inclusion of Group*LABDAY also revealed a suppression effect, such that control youth had higher morning cortisol levels on LABDAY than on HOMEDAY. See Table 4 and Figure 1.
Table 4.
Effect of Group Status by Context on Cortisol Morning Level and Slope
| df | B | S.E. | t-ratio | p-value | |
|---|---|---|---|---|---|
| For Morning Level | |||||
| Intercept | 116 | 0.494 | 0.026 | 18.895 | <0.0001 |
| Intercept*PI | 116 | 0.052 | 0.047 | 1.109 | 0.27 |
|
| |||||
| LABDAY | 362 | 0.082 | 0.039 | 2.114 | 0.035 |
| LABDAY*PI | 362 | −0.129 | 0.071 | −1.802 | 0.072 |
|
| |||||
| SCHOOLDAY | 362 | −0.07 | 0.035 | −1.991 | 0.047 |
| SCHOOLDAY*PI | 362 | 0.057 | 0.06 | 0.955 | 0.34 |
|
| |||||
| For Slope | |||||
| Intercept | 116 | −0.025 | 0.003 | −8.84 | <0.0001 |
| Intercept*PI | 116 | −0.002 | 0.004 | −0.438 | 0.662 |
|
| |||||
| LABDAY | 1857 | −0.004 | 0.004 | −1.057 | 0.291 |
| LABDAY*PI | 1857 | 0.013 | 0.006 | 1.976 | 0.048 |
|
| |||||
| SCHOOLDAY | 1857 | 0.006 | 0.003 | 1.831 | 0.067 |
| SCHOOLDAY*PI | 1857 | −0.008 | 0.005 | −1.534 | 0.125 |
|
| |||||
| CLOCKTIME | 1857 | −0.026 | 0.012 | −2.251 | 0.025 |
Note: df = degrees of freedom; B = unstandardized beta coefficient; S.E. = standard error
Note: Intercept and LABDAY effects controlled on SCHOOLDAY cortisol, SCHOOLDAY effects controlled for LABDAY cortisol. Intercept reflects cortisol levels on HOMEDAY.
Neither Stress Exposure variables were a significant predictor of LABDAY cortisol intercept or slope (ps > 0.297). The indices of prior institutionalization experience did not uniquely predict LABDAY morning cortisol or slope in the model (ps > 0.24). However, the effects of Group on LABDAY morning cortisol and slope were reduced when any of the indices of the Stress Exposure or Institutional Experience measures were included in the model (Group effect on intercept or slope ps > 0.05).
3.1.1. Are there Unique Effects of Stress Exposure or Institutionalization Experiences?
We then explored whether there were linear main effects of stress exposure or institutionalization experience on LABDAY morning cortisol and slope independent of group status (Group was not included in these models). Effects were found for Chronic Stress, Months in Institution, Number of Different Living Situations, Neglect of Basic Social Care, and Neglect of Physical Needs on cortisol slope, such that higher scores on these measures were associated with flatter slopes. Furthermore, effects were found for Months in Institution and Number of Different Living Situations on cortisol level, such that higher scores on these measures were associated with lower morning cortisol levels. No significant associations were identified between either Lifetime Adversity or Institutional Conditions Index and LABDAY morning cortisol level or slope. For more information see Table 5.
Table 5.
Effect of Stress Exposure and Institutionalization on LABDAY Cortisol Morning Level and Slope
| B | S.E. | t-ratio | p-value | |
|---|---|---|---|---|
| For Cortisol Morning Level | ||||
| Chronic Stress | −0.084 | 0.044 | −1.891 | 0.059 |
| Lifetime Stress | −0.006 | 0.014 | −0.452 | 0.651 |
| Time Spent in Orphanage | −0.003 | 0.002 | −1.996 | 0.047 |
| Number of Different Living Situations | −0.053 | 0.026 | −2.047 | 0.041 |
| Institutional Conditions Index | −0.014 | 0.011 | −1.229 | 0.22 |
| Neglect of Basic Social Care | −0.062 | 0.033 | −1.728 | 0.059 |
| Neglect of Physical Needs | −0.066 | 0.038 | −1.742 | 0.082 |
|
| ||||
| For Cortisol Slope | ||||
| Chronic Stress | 0.009 | 0.005 | 1.993 | 0.046 |
| Lifetime Stress | 0.001 | 0.001 | 0.908 | 0.364 |
| Time Spent in Orphanage | 0.000 | 0.000 | 2.307 | 0.021 |
| Number of Different Living Situations | 0.005 | 0.003 | 2.057 | 0.04 |
| Institutional Conditions Index | 0.002 | 0.001 | 1.485 | 0.138 |
| Neglect of Basic Social Care | 0.007 | 0.003 | 2.104 | 0.035 |
| Neglect of Physical Needs | 0.008 | 0.004 | 2.135 | 0.033 |
Note: B = unstandardized beta coefficient; S.E. = standard error
3.2. The Influence of Context and Institutionalization on SCHOOLDAY Diurnal Cortisol Rhythm
As reported above, we found a trend-level Level 2 main effect of SCHOOLDAY on cortisol in terms of morning level, suggesting that participants demonstrated lower morning levels of cortisol on school days. While there was no significant effect of Group * SCHOOLDAY on either the intercept or slope (ps > .10), including Group*SCHOOLDAY in the model increased the previous trend-level effect of SCHOOLDAY morning cortisol to significant (see Table 4), suggesting that control youth, but not PI youth, had significantly lower morning cortisol levels on school days compared to home days. No additional significant effects were identified after including the Stress Exposure and Institutional Experience measures into the model as Level 3 predictors (ps > 0.05). We then explored the possibility that prior experience may operate independent of group status by including the linear effects of Stress Exposure and Institutionalization Experience measures on SCHOOLDAY morning cortisol and slope (Group was not included in these models). No significant effects were identified (ps > 0.05).
3.3. The Influence of Context and Institutionalization on HOMEDAY Diurnal Cortisol Rhythm
We found no Level 2 main effect of HOMEDAY on cortisol in terms of morning level or slope nor was there any significant effect of Group * HOMEDAY on either the intercept or slope (ps < .10), suggesting that the control group and PI group demonstrated similar cortisol levels across the day. Next we wanted to examine whether the effect of Group was being suppressed by including Stress Exposure and Institutional Experience measures in the model. When Neglect of Physical Needs was included in the model, a suppression effect of Group*HOMEDAY emerged: as a group, post-institutionalized youth displayed higher morning cortisol levels when youth were at home compared to control youth (γ001 = 0.136, p = 0.045), unless they had experienced greater neglect of physical needs. If PI youth did experience high neglect of needs, the effect reversed such that post-institutionalized youth who had experienced higher levels of Neglect of Physical Needs had lower cortisol levels at home compared to control youth (γ002 = −0.070, p = 0.018; see Figure 2). A similar, but weaker, pattern was observed when we examined Neglect of Basic Social Care (PI: γ001 = 0.101, p = 0.078; Neglect of Basic Social Care: γ002 = −0.035, p = 0.035). We then explored the possibility that prior experience may operate independent of group status by including the linear effects of Stress Exposure and Institutionalization Experience measures on HOMEDAY morning cortisol and slope (Group was not included in these models). No significant effects were identified (ps > 0.05).
4.0. Discussion
The present study found that youth diurnal cortisol profiles were influenced by the context in which cortisol was collected. Post-institutionalized (PI) youth showed different cortisol effects, including both hyper- and hypo-arousal, from comparison youth—but the diverse patterns of HPA functioning that emerged within PI youth depended upon context (i.e., home or lab) and severity of early adversity experienced (i.e., Neglect of Physical Needs). The group effect of institutionalization on cortisol overlapped with variables reflecting institutionalization as well as current and lifetime stress. Exploring how the complex interplay between institutionalization, other stressful experiences, and context can differentially influence the diurnal cortisol profiles of post-institutionalized youth elucidates possible mechanisms responsible for previous, seemingly incongruent, findings.
4.1. Hyper-Arousal of the HPA Axis in Post-Institutionalized Youth
Our finding that post-institutionalized youth showed evidence of HPA hyper-arousal of cortisol levels, especially within the home context, fits with functional theories that suggest early adversity may be associated with hyper-arousal due to lack of social buffering (Doom et al., 2015; Hostinar, Johnson, & Gunnar, 2015; Struber, Struber, & Roth, 2014) or an exaggeration of the emotional/fear response to stress (Gunnar et al., 2015; Hostinar & Gunnar 2013). Cortisol functioning was specifically elevated within post-institutionalized youth in the home context, similar to our prior work within a different post-institutionalized sample that found elevated cortisol levels during a task in which youth interacted with their caregiver (Wismer Fries, Shirtcliff, & Pollak, 2008); however, we are hesitant to conclude that observing hyper-arousal implies that the home context was stressful or that these youth were “vigilant” to the home context (Del Giudice, Ellis, & Shirtcliff, 2011; Del Giudice, Ellis, & Shirtcliff, 2013). Elevated cortisol does not necessarily imply stress, per se, but instead can hint toward active engagement of the individual with their context (Shirtcliff et al., 2014). Such active engagement would not likely be needed within comparison youth at home who displayed high cortisol levels only within the laboratory (Peters et al., 2011; Eatough et al., 2009), a social context that may be engaging and arousing (e.g., Balodis, Wynne-Edwards, and Olmstead, 2010) due to novelty and unpredictability (Peters et al., 2011; Harl, Weisshuhn, and Kerschbaum, 2006).
4.2. HPA Hypo-Arousal in Post-Institutionalized Youth
Such active engagement or openness to context did not appear in the laboratory setting where, as a group, PI youth demonstrated slightly lower morning cortisol levels and flatter slopes than comparison youth. These results do not fit easily with the notion that hypo-arousal is mechanistically similar to habituation (Wust et al., 2005), as youth had not previously experienced the events of the laboratory day (e.g., MRI, Tanner staging, life stress interviews) nor do they suggest that the acute stressor failed to cross the individual’s threshold for stress activation (Andrews et al., 2007) as the added novelty and social challenge of the laboratory context resulted in blunting of cortisol functioning beyond the levels apparent in the home context. Instead, these findings are consistent with the notion that the experience of a stressor (i.e., a laboratory context) exacerbated post-institutionalized youth’s underlying propensities toward HPA axis hypo-arousal. This finding is reminiscent of prior research that supports active disengagement with an overwhelming stressor (Kiecolt-Glaser et al., 1997; Kiecolt-Glaser et al., 2003), especially severe stressors that present as a seemingly insurmountable challenge (Anisman et al., 2001; Hofer et al., 1972). Importantly, when examining the severity variables independent of group, the most severely neglected post-institutionalized youth displayed blunted HPA axis functioning across both the home and laboratory contexts. While this type of HPA axis functioning would be adaptive in adverse contexts, such as the impoverished and stressful conditions of a Romanian orphanage, it may be disadvantageous in other more supportive contexts as it inhibits their ability to be open to positive stimuli and impairs social bonding and learning (Del Giudice, Ellis, and Shirtcliff, 2011).
4.3. Reconciling Both Hyper- and Hypo-Arousal Associations with Early Adversity
The present study adds to the literature by finding evidence for both hyper- and hypo-arousal within the same study, specifically within post-institutionalized adolescents. A handful of prior studies have found HPA hyper-arousal in stress-exposed individuals, yet hypo-arousal within the most stressed individuals (Essex et al., 2011; Harkness, Stewart, and Wynne-Edwards, 2011; Laurent et al., 2014; Zalewski et al., 2012). Research with maltreated youth also finds both patterns of cortisol functioning within the same study (Bruce et al. 2009; Cicchetti and Rogosch 2001; Trickett et al., 2010). Research on parental loss and bereavement may be illuminating for understanding profiles for post-institutionalized youth as both experiences involve profound loss and familial disruption. Individuals experiencing parental loss display HPA hyper-arousal, but HPA hypo-arousal is found within the most severely stressed or at-risk individuals (Tyrka et al., 2008; Dietz et al., 2013).
Recent theoretical models describe how both hyper- and hypo-arousal may occur as a consequence of early adversity. Boyce and Ellis (2005) use a U-shaped curve to describe how elevated biological sensitivity to context (BSC) can be associated with the best and the worst of outcomes depending on early psychosocial adversity. The adaptive calibration model of stress responsivity (ACM) extended the BSC by using a cubic-shaped curve (or, alternatively, 4 ‘profiles’) to describe the relationship between stress responsivity and early adversity (Del Giudice, Ellis, and Shirtcliff, 2011; 2013). The ACM emphasizes that stress responsive physiology serves a purpose: to encode and amplify information in the environment, mediating openness of the individual to environmental inputs. When cortisol is high, the individual appears open to environmental stimuli; when low, the individual is more likely to filter non-essential information from the environment. The ACM may be helpful for framing the current study. When PI youth were faced with the additional novelty and social challenges of the laboratory stressor, they displayed hypo-arousal compared to the control youth. Such a pattern of hypo-arousal has also been shown in other studies with PI youth adopted from Eastern European or Romanian institutions (Kroupina et al. 2012; McLaughlin et al., 2015). Hypo-arousal may be necessary from a chronically stressful early environment which requires insensitivity to social challenges. The benefit of this blunted unemotional physiological pattern is that the individual is shielded from social rejection and disapproval from others, yet comes at a cost as it encourages the view that social relationships are unimportant, preventing emotional connection and sharing in the rewards of bonding (Fisher, 1998). In the home context, only the PI kids exposed to the most severe neglect displayed hypo-arousal—conversely, those exposed to lower levels of neglect demonstrated a shift toward a vigilant pattern of a highly active HPA axis. Although this appears like hyper-arousal, the benefit of high cortisol is that the individual is open to experiences and may be more readily influenced by their positive home environment.
Within post-institutionalized youth, one prior study found both HPA hyper- and hypo-arousal (Gunnar et al., 2009). That study may seem incongruent with ours in that they found hypo-arousal within moderately neglected youth and hyper-arousal within severely neglected youth. Upon closer investigation, moderate neglect was defined as being adopted before 8 months predominantly from foster care and severe neglect was being post-institutionalized for, on average, 25.5 months. Within the present study, post-institutionalized children had spent an average of 31.6 months (i.e., over two years) within the institution, and were adopted by 36 months of age on average, with several months potentially unaccounted for or with birth parents, foster parents or other living situations. Thus, the severe early life stress group described by Gunnar and colleagues (2009) may be experientially comparable to the less stressed PI youth in our study, whereas our most neglected PI youth had, unfortunately, experienced substantially greater early adversity. Albeit complicated, we contend that finding diverse, nonlinear patterns of HPA effects following adversity is common and expected for stress physiology. These findings add to a growing body of literature which identifies early adversity and risk to be associated with both hyper- and hypo-arousal of the HPA axis, and it is especially useful to observe this pattern within the same participants. This suggests hyper- and hypo-arousal are not inconsistent findings across studies, but rather reflect variations in individual differences between study participants and the context within which data are collected. These findings are consistent with recent Topological Models of adversity, which suggest that children’s biobehavioral responses to any given event will depend upon a host of factors including features of the event itself, the child’s environment, the interpersonal context surrounding the event, and pre-event individual differences (Smith & Pollak, 2020).
4.4. Study Limitations
This study has several limitations. First, we did not have specific information regarding the prenatal history or possible postnatal malnutrition, so we cannot rule out the impact of malnourishment. Future research with more precise information regarding pre- and postnatal nutrition could aid in disentangling malnourishment and parental separation. Similarly, we did not have access to the health records of parents or children, making it difficult to rule out teratogenic effects. For the same reasons, we were limited to parent report of institutionalization variables, which are subjective but are based on each parent’s first-hand experience. Second, sample size is limited to 41 post-institutionalized youth with a broad range of early adversity. We attempted to minimize this limitation by including a large sample of comparison youth, using a large number of saliva samples (up to 32 per youth) and employing a statistical technique (HLM) maximizing within- and between-individual statistical power. Third, our measures of the HPA axis are limited to cortisol levels, diurnal rhythms, and differences across contexts/days; examining cortisol reactivity could generate a more complete HPA picture (Hostinar, Johnson, & Gunnar, 2015; Koss et al., 2016; Gunnar et al., 2009; McLaughlin et al., 2015). Lastly, our study was cross-sectional, capturing a snapshot of HPA functioning years after early adversity had terminated. The strength is that the date of adoption is precisely known; nonetheless, different spans of time passed following adoption. It will be important for future studies to account for why stress responsivity patterns continue to be altered even after so many years in stable environments.
4.5. Conclusion
Early adversity in the form of institutional care affects hundreds of thousands of children and confers significant health risk (Hussey, Chang, & Kotch, 2006). The present study shows that the impact of spending the first few years of life in a setting of extreme neglect can “get under the skin” and continue to affect stress physiology, even years later. The impact on HPA functioning showed both hyper- and hypo-arousal depending on their current social context and severity of early adversity. This suggests hyper- and hypo-arousal are not inconsistent findings across studies, but rather reflect variations in individual differences between study participants and the context within which data are collected. Emerging theories emphasize that there is no “good” or “bad” cortisol profile, but rather inherent tradeoffs exist for either hyper- or hypo-arousal (Del Giudice, Ellis, and Shirtcliff, 2011; 2013; Ellis, Del Giudice, and Shirtcliff, 2012). Such calibration is adaptive, but such physiological shifts often come at a cost. Prevention efforts are of utmost importance to improve the lives of children before adversity “gets under the skin”, giving youth the chance to calibrate their stress physiology to a safe early caregiving environment and to warm and supportive social contexts across the lifespan.
Research Highlights.
On lab days, post-institutionalized (PI) youth had lower/ flatter cortisol than controls.
On home days, PI youth had higher morning cortisol than controls.
On home days, severely neglected PI youth had lower morning cortisol.
Both hyper- and hypo-cortisolism were present in PI youth, depending on the context.
Severity of neglect influence can influence cortisol in a non-linear manner.
Acknowledgements
We thank the children and families who participated in this study, and the research assistants who helped with data collection. Funding sources were NIMH MH61285 and NICHD HD03352 to S. Pollak.
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews J, Wadiwalla M, Juster RP, Lord C, Lupien SJ, and Pruessner JC. 2007. ‘Effects of manipulating the amount of social-evaluative threat on the cortisol stress response in young healthy men’, Behav Neurosci, 121: 871–6. [DOI] [PubMed] [Google Scholar]
- Anisman H, Griffiths J, Matheson K, Ravindran AV, and Merali Z. 2001. ‘Posttraumatic stress symptoms and salivary cortisol levels’, Am J Psychiatry, 158: 1509–11. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Stachowiak J, Clarren SK, and Clausen C. 2002. ‘Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population’, J Pediatr, 141: 712–7. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Wynne-Edwards KE, and Olmstead MC. 2010. ‘The other side of the curve: examining the relationship between pre-stressor physiological responses and stress reactivity’, Psychoneuroendocrinology, 35: 1363–73. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJ, Helmerhorst EJ, and Edwards KM. 2009. ‘A general enhancement of autonomic and cortisol responses during social evaluative threat’, Psychosom Med, 71: 877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, and Ellis BJ. 2005. ‘Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity’, Dev Psychopathol, 17: 271–301. [DOI] [PubMed] [Google Scholar]
- Bruce J, Davis EP, and Gunnar M. 2002. ‘Individual differences in children’s cortisol response to the beginning of a new school year’, Psychoneuroendocrinology, 27: 635–50. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, and Levine S. 2009. ‘Morning cortisol Levels in preschoolaged foster children: differential effects of maltreatment type’, Dev Psychobiol, 51: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, and Price LH. 2007. ‘Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment’, Biol Psychiatry, 62: 1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, and Price LH. 2011. ‘Effect of childhood physical abuse on cortisol stress response’, Psychopharmacology (Berl), 214: 367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, and Price LH. 2009. ‘Effect of childhood emotional abuse and age on cortisol responsivity in adulthood’, Biol Psychiatry, 66: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, and Rogosch FA. 2001. ‘Diverse patterns of neuroendocrine activity in maltreated children’, Dev Psychopathol, 13: 677–93. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, and Walker E. 2001. ‘Editorial: Stress and development: Biological and psychological consequences.’ Dev Psychopathol, 13: [PubMed] [Google Scholar]
- Cicchetti D, & Lynch M. (1995). Failures in the expectable environment and their impact on individual development: The case of child maltreatment: Risk, disorder, and adaptation. In Cicchetti D, & Cohen DJ (Eds.), Developmental psychopathology: Risk, disorder, and adaptation; (Vol. 2, pp. 32–71) 413–418. [Google Scholar]
- De Bellis MD 2001. ‘Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy’, Dev Psychopathol, 13: 539–64. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, and Shirtcliff EA. 2011. ‘The Adaptive Calibration Model of stress responsivity’, Neurosci Biobehav Rev, 35: 1562–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, and Shirtcliff EA. 2013. ‘Making sense of stress: An evolutionary-developmental framework.’ in Laviola G. and Macri S. (eds.), (Mal)adaptive aspectives of developmental stress (Springer: New York: ). [Google Scholar]
- Dickerson SS, and Kemeny ME. 2004. ‘Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research’, Psychol Bull, 130: 355–91. [DOI] [PubMed] [Google Scholar]
- Dietz LJ, Stoyak S, Melhem N, Porta G, Matthews KA, Walker Payne M, and Brent DA. 2013. ‘Cortisol response to social stress in parentally bereaved youth’, Biol Psychiatry, 73: 379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohm AA, and Gunnar MR. 2015. ‘The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence’, Psychoneuroendocrinology, 59: 102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, and Pollak SD. 2009. ‘Hormonal reactivity to MRI scanning in adolescents’, Psychoneuroendocrinology, 34: 1242–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, and Shirtcliff E. 2012. ‘Beyond allostatic load: The stress response system as a mechanism of conditional adaptation.’ in Beauchaine TP and Hinshaw SP (eds.), Child and Adolescent Psychopathology (Wiley & Sons: New York: ). [Google Scholar]
- Essex MJ, Klein MH, Cho E, and Kalin NH. 2002. ‘Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior’, Biol Psychiatry, 52: 776–84. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Kalin NH, and Armstrong JM. 2011. ‘Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence’, Dev Psychopathol, 23: 1039–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Burke HM, and Gunnar MR. 2008. ‘Salivary cortisol levels in children of low-income women with high depressive symptomatology’, Dev Psychopathol, 20: 423–36. [DOI] [PubMed] [Google Scholar]
- Fisher HE 1998. ‘Lust, attration, and attachment in mammalian reproduction’, Human Nature, 9: 23–52. [DOI] [PubMed] [Google Scholar]
- Fisher PA, and Stoolmiller M. 2008. ‘Intervention effects on foster parent stress: associations with child cortisol levels’, Dev Psychopathol, 20: 1003–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, and Burraston BO. 2007. ‘Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity’, Psychoneuroendocrinology, 32: 892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Van Ryzin MJ, and Gunnar MR. 2011. ‘Mitigating HPA axis dysregulation associated with placement changes in foster care’, Psychoneuroendocrinology, 36: 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, and Hellhammer DH 2005. A new view on hypocortisolism. Psychoneuroendocrinology, 30: 1010–1016. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Bruce J, and Grotevant HD. 2000. ‘International adoption of institutionally reared children: research and policy’, Dev Psychopathol, 12: 677–93. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, and Van Ryzin MJ. 2009. ‘Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children’, Psychoneuroendocrinology, 34: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, and Schuder M. 2001. ‘Salivary cortisol levels in children adopted from romanian orphanages’, Dev Psychopathol, 13: 611–28. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, and Vazquez DM. 2001. ‘Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development’, Dev Psychopathol, 13: 515–38. [DOI] [PubMed] [Google Scholar]
- Gunnar, Megan R, Hostinar Camelia E, Sanchez Mar M, Tottenham Nim, and Sullivan Regina M. 2015. ‘Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models’, Social neuroscience, 10: 474–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, and Pollak SD. 2013. ‘Early neglect is associated with alterations in white matter integrity and cognitive functioning’, Child Dev, 84: 1566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, and Wynne-Edwards KE. 2011. ‘Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment’, Psychoneuroendocrinology, 36: 173–81. [DOI] [PubMed] [Google Scholar]
- Harl B, Weisshuhn S, and Kerschbaum HH. 2006. ‘Cortisol titre increases with novelty of academic oral examinations’, Neuro Endocrinol Lett, 27: 669–74. [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, and Johnson DE. 2008. ‘The International Adoption Project: population-based surveillance of Minnesota parents who adopted children internationally’, Matern Child Health J, 12: 162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Wolff CT, Friedman SB, and Mason JW. 1972. ‘A psychoendocrine study of bereavement. II. Observations on the process of mourning in relation to adrenocortical function’, Psychosom Med, 34: 492–504. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. 1975. “Four-factor index of social position.” In. Yale University, Department of Sociology. [Google Scholar]
- Hostinar CE, and Gunnar MR. 2013. ‘Future directions in the study of social relationships as regulators of the HPA axis across development’, J Clin Child Adolesc Psychol, 42: 564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, and Gunnar MR 2015. ‘Early social deprivation and the social buffering of cortisol stress responses in late childhood: An experimental study’, Developmental Psychology, 51: 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey JM, Chang JJ, and Kotch JB. 2006. ‘Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences’, Pediatrics, 118: 933–42. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, and Jacobs WJ. 2006. ‘Stress differentially modulates fear conditioning in healthy men and women’, Biol Psychiatry, 59: 516–22. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Bruce J, Tarullo AR, and Gunnar MR. 2011. ‘Growth delay as an index of allostatic load in young children: predictions to disinhibited social approach and diurnal cortisol activity’, Dev Psychopathol, 23: 859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE 2000. ‘Medical and developmental sequelae of early childhod institutionalization in Eastern European adoptees.’ in Nelson CA (ed.), The effects of early adversity on neurobehavioral development (Erlbaum Associates: Mahway, NJ: ). [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, and Davidson RJ. 2008. ‘Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor’, Psychoneuroendocrinology, 33: 517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, and Malarkey WB. 2003. ‘Love, marriage, and divorce: newlyweds’ stress hormones foreshadow relationship changes’, J Consult Clin Psychol, 71: 176–88. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, and Malarkey WB. 1997. ‘Marital conflict in older adults: endocrinological and immunological correlates’, Psychosom Med, 59: 339–49. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, and Zahn-Waxler C. 2001. ‘Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges’, Dev Psychopathol, 13: 695–719. [DOI] [PubMed] [Google Scholar]
- Koob GF, and Le Moal M. 2008a. ‘Addiction and the brain antireward system’, Annu Rev Psychol, 59: 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF 2008b. ‘Review. Neurobiological mechanisms for opponent motivational processes in addiction’, Philos Trans R Soc Lond B Biol Sci, 363: 3113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss, Kalsea, Hostinar Camelia E, Bonny Donzella, and Gunnar Megan R. 2014. ‘Social deprivation and the HPA axis in early development’, Psychoneuroendocrinology, 50: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss, Kalsea J, Mliner Shanna B, Donzella Bonny, and Gunnar Megan R. 2016. ‘Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children’, Psychoneuroendocrinology, 66: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupina MG, Fuglestad AJ, Iverson SL, Himes JH, Mason PW, Gunnar MR, Miller BS, Petryk A, and Johnson DE. 2012. ‘Adoption as an intervention for institutionally reared children: HPA functioning and developmental status’, Infant Behav Dev, 35: 829–37. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, and Leve LD. 2014. ‘Stress system development from age 4.5 to 6: family environment predictors and adjustment implications of HPA activity stability versus change’, Dev Psychobiol, 56: 340–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, and Taylor SF. 2007. ‘Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli’, Am J Psychiatry, 164: 1250–8. [DOI] [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Westerlund A, Pollak SD, Nelson CA, and Gunnar MR. 2013. ‘The effect of early deprivation on executive attention in middle childhood’, J Child Psychol Psychiatry, 54: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu M, Buss C, Walker D, Pruessner J, and McEwen BS. 2006. ‘Beyond the stress concept: Allostatic Load--A developmental biological and cognitive perspective.’ in Cicchetti D. and Cohen D. (eds.), Developmental Psychopathology (John Wiley & Sons: Hoboken, NJ: ). [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, and Schmidt LA. 2009. ‘Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project’, Biol Psychiatry, 66: 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, and Wingfield JC. 2003. ‘The concept of allostasis in biology and biomedicine’, Horm Behav, 43: 2–15. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, and Nelson CA 3rd. 2015. ‘Causal effects of the early caregiving environment on development of stress response systems in children’, Proc Natl Acad Sci U S A, 112: 5637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, and Zhou ES. 2007. ‘If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans’, Psychol Bull, 133: 25–45. [DOI] [PubMed] [Google Scholar]
- Miller LC, Chan W, Litvinova A, Rubin A, Tirella L, and Cermak S. 2007. ‘Medical diagnoses and growth of children residing in Russian orphanages’, Acta Paediatr, 96: 1765–9. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, and Buss K. 1996. ‘Behavioral inhibition and stress reactivity: the moderating role of attachment security’, Child Dev, 67: 508–22. [PubMed] [Google Scholar]
- Nicolson NA, Davis MC, Kruszewski D, and Zautra AJ. 2010. ‘Childhood maltreatment and diurnal cortisol patterns in women with chronic pain’, Psychosom Med, 72: 471–80. [DOI] [PubMed] [Google Scholar]
- Peters S, Cleare AJ, Papadopoulos A, and Fu CH. 2011. ‘Cortisol responses to serial MRI scans in healthy adults and in depression’, Psychoneuroendocrinology, 36: 737–41. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, and Boxer A. 1988. ‘A self-report measure of pubertal status: Reliability, validity, and initial norms’, Journal of Youth and Adolescence, 17: 117–33. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, and Lupien SJ. 2010. ‘Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner’, Psychoneuroendocrinology, 35: 179–91. [DOI] [PubMed] [Google Scholar]
- Quas Jodi-A, Murowchick Elise, Bensadoun Jennifer, and homas Boyce W. 2002. ‘Predictors of children’s cortisol activation during the transition to kindergarten.’, Journal of Developmental and Behavioral Pediatrics, 23: 304–13. [DOI] [PubMed] [Google Scholar]
- Quevedo K, Johnson A, Loman M, Lafavor T, and Gunnar M. 2012. ‘The Confluence of Adverse Early Experience and Puberty on the Cortisol Awakening Response’, Int J Behav Dev, 36: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, and Flynn M. 2007. ‘Childhood adversity and youth depression: influence of gender and pubertal status’, Dev Psychopathol, 19: 497–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, and Hammen C. 1999. ‘Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective’, Child Dev, 70: 660–77. [DOI] [PubMed] [Google Scholar]
- Rutter M, and O’Connor TG. 2004. ‘Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees’, Dev Psychol, 40: 81–94. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, and Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev, 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, and Pollak SD. 2009. ‘Pubertal development: correspondence between hormonal and physical development’, Child Dev, 80: 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, and Phan JM. 2014. ‘Hormones: commentary. Riding the physiological roller coaster: adaptive significance of cortisol stress reactivity to social contexts’, J Pers Disord, 28: 40–51. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Skinner ML, Obasi EM, and Haggerty KP. 2017. ‘Positive parenting predicts cortisol functioning six years later in young adults’, Dev Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, and Zahn-Waxler C. 2009. ‘Neurobiology of empathy and callousness: implications for the development of antisocial behavior’, Behav Sci Law, 27: 137–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren E, Leanderson P, and Kristenson M. 2006. ‘Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-aged Swedish men and women’, Int J Behav Med, 13: 193–200. [DOI] [PubMed] [Google Scholar]
- Smith KE, and Pollak SD. In Press. Re-thinking concepts and categories for understanding the neurodevelopmental effects of early childhood adversity. Perspect Psychol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struber N, Struber D, and Roth G. 2014. ‘Impact of early adversity on glucocorticoid regulation and later mental disorders’, Neurosci Biobehav Rev, 38: 17–37. [DOI] [PubMed] [Google Scholar]
- Susman EJ 2006. ‘Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis’, Neurosci Biobehav Rev, 30: 376–89. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, and Gunnar MR. 2006. ‘Child maltreatment and the developing HPA axis’, Horm Behav, 50: 632–9. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, and Lieberman MD. 2008. ‘Neural bases of moderation of cortisol stress responses by psychosocial resources’, J Pers Soc Psychol, 95: 197–211. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, and Putnam FW. 2010. ‘Attenuation of cortisol across development for victims of sexual abuse’, Dev Psychopathol, 22: 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, and Carpenter LL. 2008. ‘Childhood parental loss and adult hypothalamic-pituitary-adrenal function’, Biol Psychiatry, 63: 1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watch, Human Rights. 1998. Abandoned to the State: Cruelty and Neglect in Russian Orphanages (New York, NY: ). [Google Scholar]
- Wiik KL, Loman MM, Van Ryzin MJ, Armstrong JM, Essex MJ, Pollak SD, and Gunnar MR. 2011. ‘Behavioral and emotional symptoms of post-institutionalized children in middle childhood’, J Child Psychol Psychiatry, 52: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer Fries AB, Shirtcliff EA, and Pollak SD. 2008. ‘Neuroendocrine dysregulation following early social deprivation in children’, Dev Psychobiol, 50: 588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Federenko IS, van Rossum EF, Koper JW, and Hellhammer DH. 2005. ‘Habituation of cortisol responses to repeated psychosocial stress-further characterization and impact of genetic factors’, Psychoneuroendocrinology, 30: 199–211. [DOI] [PubMed] [Google Scholar]
- Yen IH, and Syme SL. 1999. ‘The Social Environment and Health: A Discussion of the Epidemiologic Literature’, Annual Reviews in Public Health, 20: 287–308. [DOI] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Kiff CJ, and Fisher PA. 2012. ‘Understanding the relation of low income to HPA-axis functioning in preschool children: cumulative family risk and parenting as pathways to disruptions in cortisol’, Child Psychiatry Hum Dev, 43: 924–42. [DOI] [PMC free article] [PubMed] [Google Scholar]