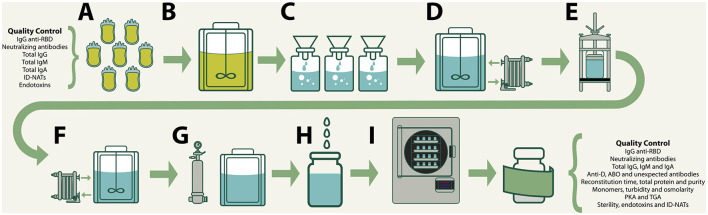
Figure 1.
Production of VP-IVIg and CP-IVIg by the method of caprylic acid precipitation: (A) Collection, testing and approval of raw plasma. (B) Precipitation of plasma proteins by addition of caprylic acid 5–6% (v/v). (C) Removal of precipitates by gravity filtration. (D) Diafiltration, concentration and formulation. (E) Strong anion exchanger chromatography. (F) Readjustment of the formula. (G) Sterilizing filtration. (H) Filling in type 1 borosilicate vials (40 mL/vial). (I) Lyophilization.