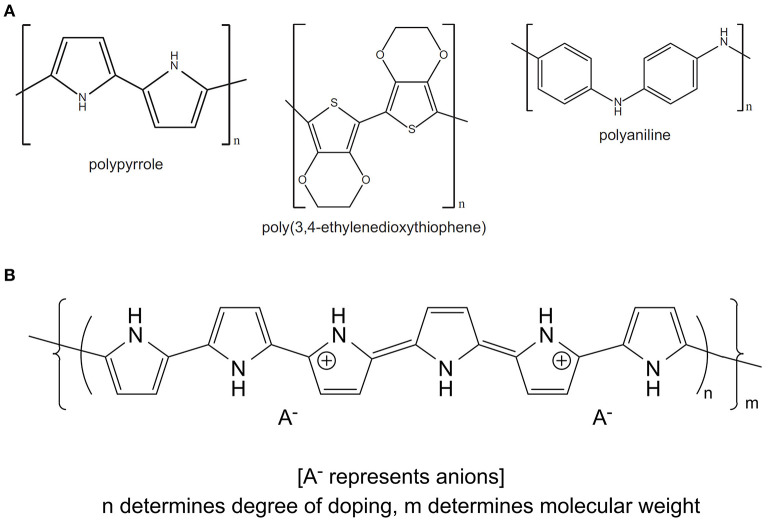
Figure 1.
(A) Chemical structures of the common OCPs polypyrrole, poly(3,4-ethylenedioxythiophene) and polyaniline. (B) Schematic illustrating the incorporation of a dopant anion during oxidation of monomeric species to form the OCP conjugated backbone (PPy). (B) Reproduced with permission from Gilmore et al. (27).