Figure 2.
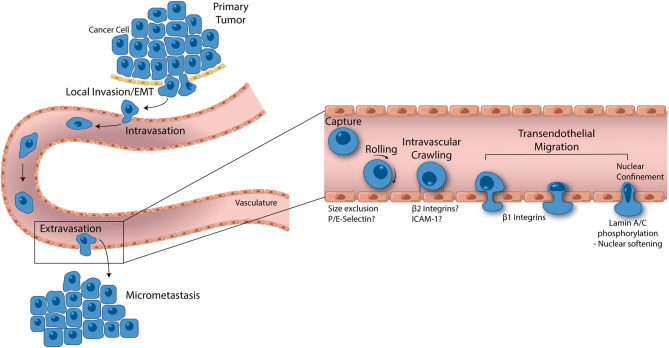
From local invasion to micrometastasis formation, metastatic cancer cells take a complex, and strenuous path to complete the metastasis cascade. While the percentage of cancer cells able to successfully form a secondary tumor is low, metastasis accounts for 90% of cancer mortality, thus making it one of the most important oncological processes that can be exploited for drug discovery. Cancer cells begin the metastasis cascade by transitioning from a static, epithelial phenotype to a dynamic, mesenchymal phenotype (EMT), and invading surrounding tissue. Once on the basal endothelial cell surface, metastatic cancer cells may intravasate into circulation, moving across the endothelium from the abluminal to luminal surface. Shear blood flow releases the cancer cell from the luminal vascular wall where it is carried to a secondary location. Size restriction in the capillaries halts the cancer cell, where β1 integrins can attach and facilitate transendothelial migration. In some cases, P and E selectin as well as β2 integrins have been implicated in cancer cell extravasation, however, more studies are needed to understand the role these proteins play. Given the ridged nature of the cell nucleus, all contents of a transmigrating cancer cell are pushed into the basement membrane until the last minute when which the nucleus is squeezed through. Lamin A/C phosphorylation permits nuclear softening and severe deformation, aiding in the push across the endothelium. Successful transendothelial migration constitutes the final step in forming a secondary tumor. In vitro models have greatly contributed to these fresh understandings of metastasis and are opening the door to novel drug targets.