Figure 3.
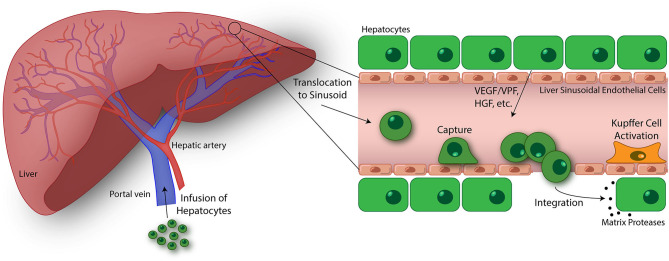
Emerging topics in the study of transendothelial migration (TEM). Hepatocyte transplantation is a promising treatment for liver disease and failure. In this process, donor hepatocytes are perfused into the portal vein where they may translocate to the liver sinusoids. Similar to cancer cells, size restrictions in the liver microvasculature, as well as potential integrin interactions, trap the hepatocytes at the apical endothelial cell surface. Entrapment resulting in ischemia-reperfusion events and Kupffer cell activation lead to liver sinusoidal endothelium disruption. Additionally, vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF), hepatocyte growth factor (HGF), and other factors released by native hepatocytes in the liver plate further drive endothelium disruption, permitting passage of transfused hepatocytes. Once across, donor hepatocytes integrate with native hepatocytes as mediated through the activation of matrix proteases and separation of gap junctions (146). The ultimate goal of this process is to engraft these donor hepatocytes into the liver parenchyma and restore liver function. While there has been some success using this treatment strategy, an improved understanding of the hepatocyte TEM process may lead to discoveries promoting enhanced engraftment of hepatocytes in the liver. Using in vitro devices to breakdown and study this process is key to this progress.