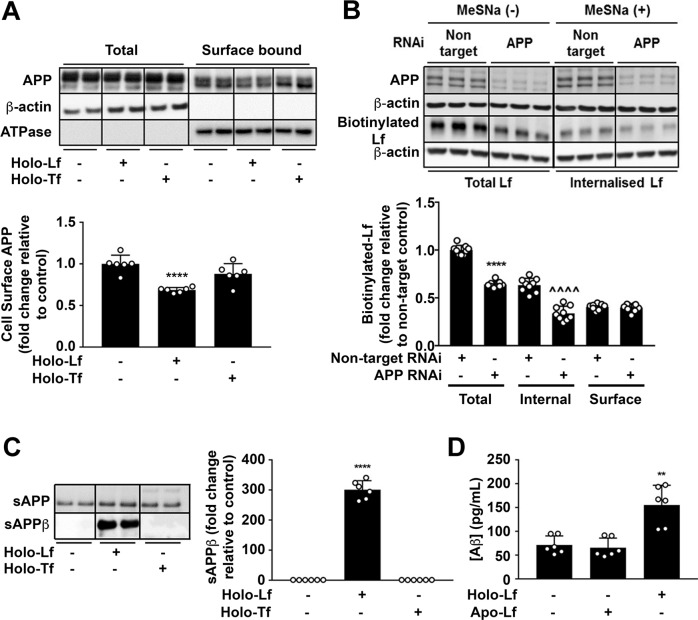
Fig. 2. Iron-bound Lf decreases cell-surface APP levels and promotes the amyloidogenic processing of APP.
A Biotinylation of surface proteins on primary murine neurons cultured in holo-Lf or holo-Tf (500 nM; 2 h) and followed by streptavidin immunoprecipitation shows a decrease in biotinylated APP with only holo-Lf when normalised against Na+/K+ ATPase surface protein content. B Biotinylated holo-Lf (200 nM; 1 h at 37 °C) was added to SH-SY5Y cells transfected with control non-target or APP RNAi (20 nM for 48 h) before being subjected to the ligand internalisation assay. APP depletion was confirmed by western blot (22C11). Total biotinylated holo-Lf (MeSNa (–)) and internalised holo-Lf (MeSNa (+)) (detected by Streptavidin-HRP) were quantified whilst surface-bound biotinylated Lf was determined by subtracting the internal from total fraction. C Primary murine neurons treated with holo-Lf or holo-Tf (500 nM; 2 h) were evaluated for sAPPβ release into the media. D Aβ production was also measured by ELISA on the media after treatment with apo- or holo-Lf (500 nM; 2 h). Data are mean ± SEM of three separate experiments performed in duplicate (A, B, D) or triplicate (C). Quantified data depict fold change compared to non-treated control cells, **p < 0.01 and ****p < 0.0001 or in (B) the non-targeted internal fraction, ^^^^p < 0.0001, as analysed statistically by two-tailed t-tests.